Collaborators at the Department of Energy's Oak Ridge National Laboratory and U.S. universities used neutron scattering and other advanced characterization techniques to study how a prominent catalyst enables the "water-gas shift" reaction to purify and generate hydrogen at industrial scale.
Results published in the Journal of the American Chemical Society settled a long-standing debate about the catalyst's reaction mechanism, opening routes to improve the cost and efficiency of large-scale hydrogen production.
"Our work significantly advances fundamental understanding of a complex, industry-critical catalyst that has been difficult to study," said Zili Wu of ORNL's Chemical Sciences Division. "Determining how this reaction works at the atomic level enables further efforts to optimize the catalyst for improved performance."
Collaborators investigated a copper-chromium-iron oxide catalyst (CuCrFeOx) provided by Lehigh University.
"We already know the existing CuCrFeOx catalyst works, but how it works has been the subject of debate," said ORNL's Felipe Polo-Garzon, who worked with Wu on the team's multimodal approach to pinpoint the catalyst's reaction mechanism.
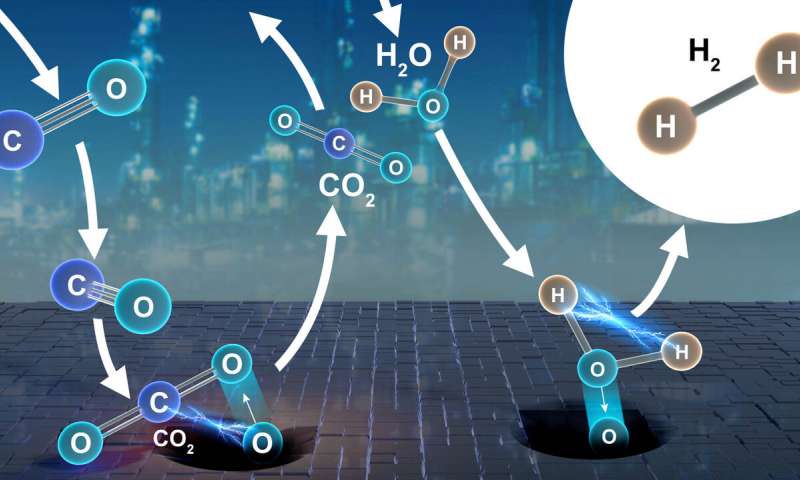
The goal was to study how the catalyst behaves in real-world conditions to find evidence of either an oxidation-reduction ("redox") or an associative mechanism—two predominant theories about how CuCrFeOx works to produce hydrogen.
In a redox reaction, reactants exchange some of their atoms with the catalyst's surface to yield new substances, in this example, hydrogen and carbon dioxide. By contrast, in an associative reaction, all the reacting molecules bond to the catalyst's surface in an intermediate step to arriving at the final products.
To prove unambiguously how the CuCrFeOx catalyst works (redox vs. associative mechanism), researchers cast a wide net of experimental and computational methods.
All results pointed to the same conclusion—a redox reaction. At high-temperature conditions, the catalyst loses oxygen atoms to make room for water molecules that dissociate and give off pure hydrogen.
"The answer is important because it helps us identify the critical point in the reaction where hydrogen is generated," said Polo-Garzon.
Many existing catalysts have been created through trial and error, which often limits their efficiency. The team's fundamental discovery could eliminate guesswork and tell researchers precisely where to look for opportunities to synthesize a better catalyst for generating hydrogen.
Hydrogen is the earth's most abundant element, but it does not occur naturally in the pure form needed by industries for oil refining, ammonia production for fertilizers, food processing, metal treatment, and other broad applications. Most of the world's hydrogen supply is produced by steam methane reforming—converting natural gas into a hydrogen mixture that is refined via water-gas shift catalysis to make pure hydrogen.





