Researchers in Spain have developed hydrogen production without contact electrodes via water electrolysis mediated by the microwave-triggered redox activation of solid-state ionic materials at low temperatures (< 250 °C). A paper on their work is published in Nature Energy.
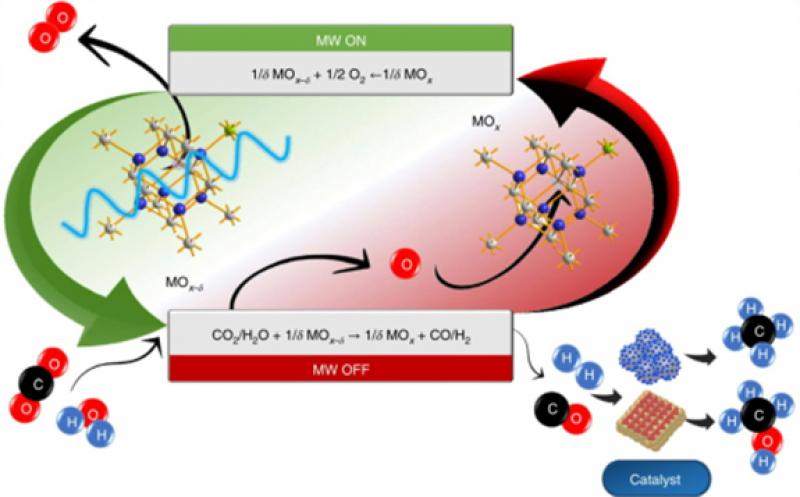
Schematic illustration of the microwave-induced redox cycle. Microwave irradiation of doped ceria materials induce its reduction and triggers the release of gaseous oxygen. The input of a suitable sweep gas on microwave switching-off leads to the material reoxidation via gas deoxygenation and the formation of valuable molecular energy carriers. Serra et al.
Water was reduced via reaction with non-equilibrium gadolinium-doped CeO2 that was previously in situ electrochemically deoxygenated by the sole application of microwaves. The microwave-driven reduction was identified by an instantaneous electrical conductivity rise and O2 release.
First, microwaves interact with the crystalline oxide, leading to an instantaneous rise in electrical conductivity that is accompanied by the material reduction (deoxygenation).
The second step involves splitting water through a spontaneous reaction with the activated material, which leads to direct H2 formation and reoxygenation of the material.
This process was cyclable; H2 yield and energy efficiency were material- and power-dependent. Deoxygenation of low-energy molecules (H2O or CO2) led to the formation of energy carriers and enabled CH4 production when integrated with a Sabatier reactor.
The researchers suggested that this method could be extended to other reactions such as intensified hydrocarbons synthesis or oxidation.