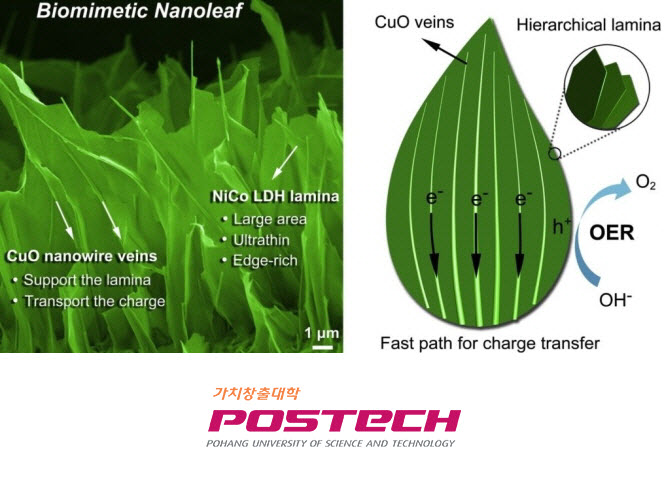
Light is used to make nutrients, and oxygen is produced on small green leaves. The leaves, also called green plants, divide the nutrients produced by photosynthesis into tissues to feed the plants, and release oxygen to make all living things alive. Recently, ‘Biomimetic nanoleaf electrocatalyst’, which can produce hydrogen energy, which is an eco-friendly clean energy, has been developed by mimicking the photosynthesis mechanism of natural leaves.
Dr. Chen Bin, Ph.D., in the Department of Chemical Engineering, has succeeded in developing a ‘nanoleaf catalyst’ that mimics the leaves of a monocotyledonous plant and promotes oxygen generation during water decomposition to produce hydrogen energy. The nanoleaf catalyst developed this time is confirmed to have improved catalytic activity up to 9.3 times than the existing catalyst.
In order to obtain hydrogen energy, water must be decomposed into oxygen and hydrogen. The water decomposition reaction is carried out by a hydrogen generating catalyst and an oxygen generating catalyst reaction. Among them, the core technology is to develop an oxygen generating catalyst which is relatively difficult to react.
The team focused on photosynthesis by efficiently delivering moisture and nutrients to the leaf veins, which are mostly annual leaves, and developed nano leaves that mimic the advantages of natural leaves. It is synthesized ‘layered double hydroxide (LDH)’ that mimics the leaf body structure that causes photosynthesis on ‘CuO nanowire’ modeled after leaf vein that delivers water and nutrients. The nanoleaf catalyst promotes the oxygen generation reaction by using a large surface area of the leaf body, that is, the LDH plate structure, and greatly improves the oxygen generation efficiency by rapidly transporting charges by the leaf wire nanowires. In addition, it appears to be stable and flexible, and thus has high utility.
Professor Jeon Baong-joong said, “The development of nanoleaf catalysts has greatly facilitated the most difficult oxygen-producing reactions in water decomposition reactions.” “We will be able to use it for hydrogen energy production through water decomposition in the future.” Said.
Meanwhile, this research was conducted as part of the Korea Research Foundation’s support for mid-sized research projects in the basic science field in 2019, supported by the Ministry of Science, ICT and Education.