Researchers at Ariel University in Israel have developed a new type of hydrogen generator for “on-demand” use with fuel cells. Hydrogen is produced in a catalytic hydrolysis reaction of sodium borohydride (NaBH4) with ruthenium powder as a catalyst. The proposed generator is portable and lightweight; has high energy density; is easy to use, refill, and clean; and is designed for long working periods with the capability for restart after prolonged rests.
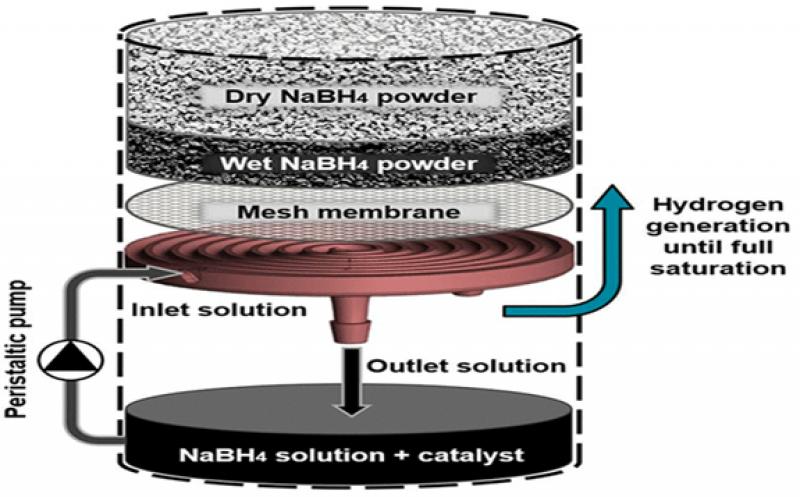
The system consists of two main chambers: an upper chamber with granulated sodium borohydride powder and a lower reaction chamber with a solution of water and catalyst. A peristaltic pump regulates the flow of solution through a spiral channel which is situated in the solids chamber.
The powder is separated from the channel by a mesh which prevents its clogging. The solution becomes saturated while flowing through the channel and drips into the reaction chamber. To maintain constant H2 flow rate levels, the solution temperature and H2 pressure in the reaction chamber are constantly controlled.
This system is significantly lighter and requires much less operating energy than other similar generators, the researchers said.
In a paper published in the ACS journal Energy & Fuels, the team reported that during 6.3 hours of their experiment, 110 L of hydrogen was generated with an average flow rate of 290 mL/min and 98% conversion efficiency. The achieved energy density is 1300 Wh/kg for the fuel, 540 Wh/kg for the generator, and 377 Wh/kg with an attached 30 W fuel cell.