Researchers at the Chinese University of Hong Kong (CUHK) have developed a sulfur-based redox flow battery that is claimed to be able to operate for 15 consecutive hours of runtime and for over 2,000 hours' cycling without obvious capacity decay.
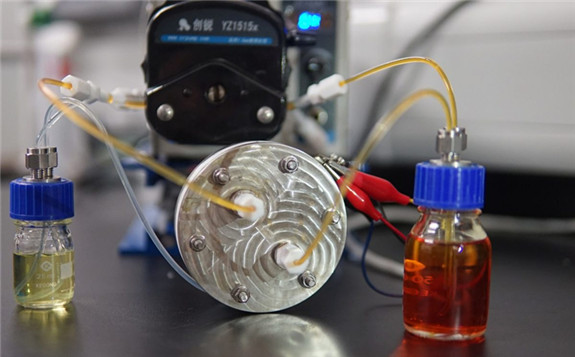 The demonstrator polysulfide-iodide redox flow battery. Image: Chinese University of Hong Kong (CUHK)
The demonstrator polysulfide-iodide redox flow battery. Image: Chinese University of Hong Kong (CUHK)
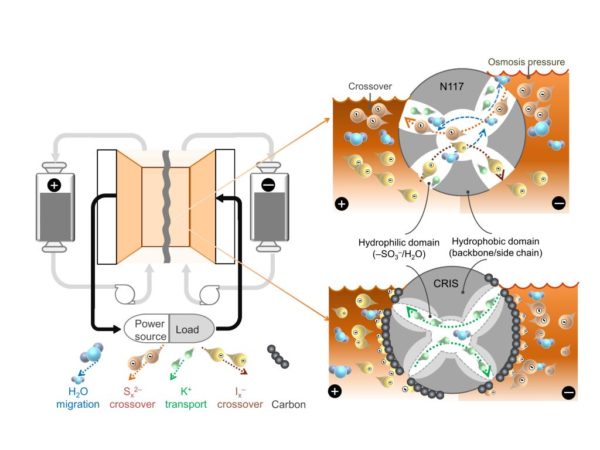
This performance, according to the scientists, was achieved by applying a novel charge-reinforced, ion-selective (CRIS) membrane to a polysulfide-iodide redox flow battery built by the same research team in 2016. “Sulphur-based redox flow batteries adopting a commercial ion-selective membrane are compromised by the crossover and precipitation of active materials, leading to a rapid capacity decay and poor lifetime, and its application in large scale energy storage fails to be effective,” they emphasized.
The membrane was fabricated with a polymer-bound carbon and placed onto commercial Nafion membrane N117 to keep the two electrodes apart. Nafion membranes are proton-conductive polymer films that are used in redox flow batteries to prevent a problem known as “crossover,” which occurs during charging and recharging when battery electrolyte components cross the membrane in the battery cell, thus causing capacity losses that can reach up to 50%.
“The absorption of negatively charged species in porous carbon has strengthened the negative charge of the membrane and reduced the loss of active materials, which dramatically increased the stability and the lifetime of batteries,” the scientists said, referring to the use of the CRIS membrane.
The redox flow battery also exhibited a capacity decay rate of just 0.005% per day for 1,200 cycles, and a lifetime with over 2,000 hours' cycling, which the academics said corresponds to approximately three months. For comparison, a device built exclusively with commercial membrane N117 has a lifetime of 160 hours' cycling, or 6.7 days.
“Under the condition in which the energy storage device with a new battery continuously operated and discharged for more than 15 hours, the surprising result yielded, in turn, a levelized cost of storage (LCOS) for this system which is competitive with other state-of-the-art redox flow techniques for long-duration energy storage applications,” the scientists concluded. “This approach can be universally applied in other redox flow batteries using sulphur-bromine or sulphur-iron materials, and in organic redox flow batteries, which goes further, to fulfill a high stability of cycling.”
The novel membrane is described in the study Polysulfide-based redox flow batteries with long life and low levelized cost enabled by charge-reinforced ion-selective membranes, published in nature energy.