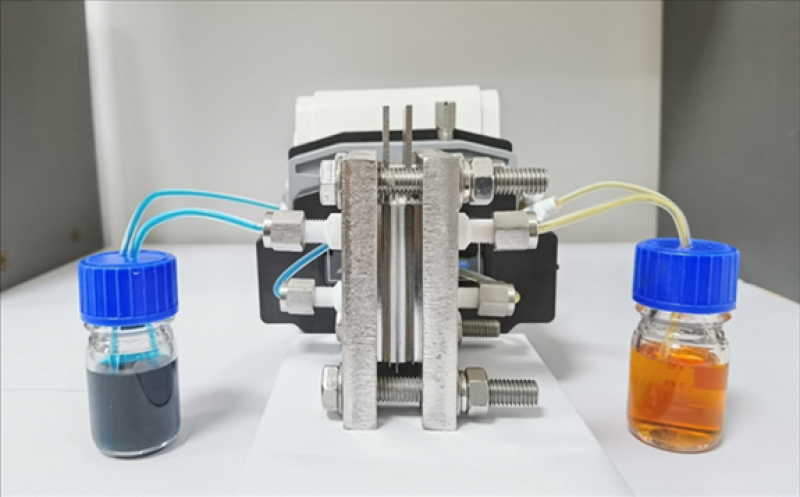
Scientists from the South China University of Technology have built an aqueous redox flow battery with a positive electrolyte made of the electroactive organic molecule called TEMPO, which they describe as a chemical compound with easily reversed oxidation states and a high potential for energy.
Also known by the formula (CH2)3(CMe2)2NO, TEMPO is a well-known electroactive aminoxyl radical used in several applications by organic chemists, polymer scientists and molecular biologists. It consists of a bright orange solid that has a stable free radical nature and can be stored for years.
“However, TEMPO cannot be directly applied to aqueous redox flow batteries due to the high hydrophobicity of the molecular skeleton,” the research team said, adding that, when it is left unmodified, it is unable to dissolve in the liquid needed to facilitate the energy exchange in flow batteries. “We developed a strategy to functionalize TEMPO with viologen, an organic compound that has highly reversible redox reactions, to improve TEMPO’s hydrophilicity.”
The TEMPO derivative the scientists developed is claimed to promote the battery's aqueous solubility, as viologen is highly water-soluble, and also to increase redox potential due to the strong electron-withdrawing effect of viologen itself.
Furthermore, viologen's densely positive charge minimizes so-called “crossover.” This phenomenon, which can cause capacity losses that can reach up to 50%, occurs during charging and recharging, when battery electrolyte components cross the membrane in the battery cell and the redoxmers – which are redox-active molecules that can store energy in the batteries' electrolytes – migrate to the wrong side of the device.
Moreover, the salt nature of viologen endows TEMPO with a decent conductivity in an aqueous solution, the Chinese group explained.
The viologen-modified TEMPO was tested in an aqueous redox flow battery and was found to help the device retain a capacity of 99.98% per cycle, which they describe as sufficient to make the battery hold nearly all its stored energy when not in active use.
“This work overcomes the disadvantages of TEMPO by viologen-functionalization and realizes its application in aqueous redox flow battery,” said the research corresponding author, Zhenxing Liang. “The molecular design concept provides a strategy for novel organic electroactive materials and lays a foundation for the application of [an] aqueous organic flow battery.”
The process to synthesize a molecular compound that serves as a low-cost electrolyte for the battery is described in the paper Viologen-Decorated TEMPO for Neutral Aqueous Organic Redox Flow Batteries, published in Energy Material Advances.