A new type of catalyst breaks down polyolefin plastics into new, useful products. This project is part of a new strategy to reduce the amount of plastic waste and its impact on our environment, as well as recover value that is lost when plastics are thrown away. The catalyst was developed by a team from the Institute for Cooperative Upcycling of Plastic (iCOUP), a U.S. Department of Energy, Energy Frontier Research Center. The effort was led by Aaron Sadow, the director of iCOUP, scientist at Ames National Laboratory, and professor at Iowa State University; Andreas Heyden, professor at the University of South Carolina; and Wenyu Huang, scientist at Ames Lab and professor at Iowa State. The new catalyst is made only of earth-abundant materials, which they demonstrated can break carbon-carbon (CC) bonds in aliphatic hydrocarbons.
Aliphatic hydrocarbons are organic compounds made up of only hydrogen and carbon. Polyolefin plastics are aliphatic hydrocarbon materials composed of long chains of carbon atoms linked together to form strong materials. These materials are a big part of the plastic waste crisis. Wenyu Huang said, “More than half of produced plastics so far are polyolefin based.”
Polyolefin plastics are used everywhere in the modern world, including in shrink wrap and other packaging products, containers for liquids such as detergents or milk, fibers in waterproof clothing, dental floss, and electronics. Yet, as Andreas Heyden explained, polyolefins are some of the most difficult plastics to recycle and new approaches are needed. One such promising alternative to recycling is known as upcycling. This approach involves chemical transformation of the materials into higher value products.
One way to upcycle polyolefins is a chemical process called hydrogenolysis. During this process, a catalyst splits chains of molecules by cutting CC bonds and adding hydrogen. According to Aaron Sadow, catalysts that are used for hydrogenolysis are typically based on precious metals, such as platinum. Platinum is expensive because of its low abundance in the earth’s crust, and due to its effectiveness, it is used in many types of catalytic transformations.
To address both challenges of sustainability and economy, Heyden said, “We thought we’d be able to use earth-abundant elements to create much cheaper catalytic materials, and by assembling these elements in a certain way we might achieve a high selectivity and still very good activity.”
The team discovered that zirconia, an earth-abundant metal oxide, can cut CC bonds in aliphatic hydrocarbon polymers at about the same speed of precious metal catalysts. “We were surprised that we could do hydrogenolysis of CC bonds, using zirconium oxide as the catalyst. The conventional paradigm is that zirconia is not very reactive on its own,” said Sadow.
The key to its success is the structure of the catalyst, which was designed by Wenyu Huang and his group. “In this architecture, ultrasmall zirconia nanoparticles are embedded between two plates of mesoporous silica. The two silica plates are fused, with the zirconia embedded in the middle, like a sandwich,” Huang said. “The pores in the silica provide access to the zirconia, while the sandwich-like structure protects the zirconia nanoparticles from sintering or crystallization, which would make them less effective.”
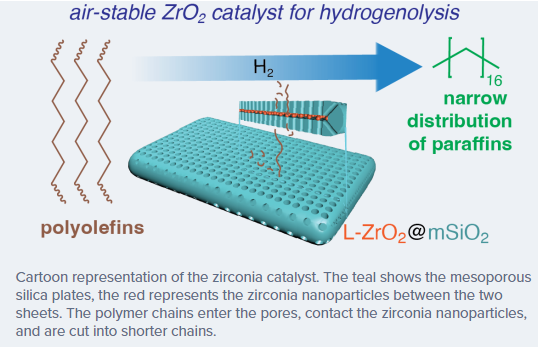
Heyden’s team was in charge of modeling the reaction and understanding where and how the active site works under reaction conditions. “And so for that we do both quantum chemical modeling of the catalyst and the chemical reactions together with some classical chemical reactor modeling,” he explained. “And here we really saw the importance of that amorphous zirconia structure.”
According to Sadow, the idea to study zirconia in hydrogenolysis was based on previous pioneering research of polymer depolymerization using zirconium hydrides studied in the late 1990s. “Harnessing zirconium hydrides for hydrogenolysis is really nice chemistry,” he said. “The problem is those zirconium organometallic species are really air and water sensitive. So they have to be handled under the cleanest of conditions. Typically polymer waste is not pure and isn’t supplied as a clean and perfectly dry starting material. Using a zirconium hydride catalyst, you'd have to really worry about impurities that inhibit the chemistry.”
The new zirconia material the team developed is simply heated under vacuum before the reactions, and it stays active during the hydrogenolysis process. “Zirconium oxide is easily handled in air and then activated. It doesn't require any kind of really specialized conditions, which was also exciting,” Sadow said. “Being able to take an air-exposed metal oxide, heat it with an alkane, and generate an organometallic is a really powerful reaction that enables this kind of hydrogenolysis process. It potentially could enable lots of interesting catalytic transformations of hydrocarbons that were previously not considered.”
This research is further discussed in the paper “Ultrasmall amorphous zirconia nanoparticles catalyse polyolefin hydrogenolysis,” written by Shaojiang Chen, Akalanka Tennakoon, Kyung-Eun You, Alexander L. Paterson, Ryan Yappert, Selim Alayoglu, Lingzhe Fang, Xun Wu, Tommy Yunpu Zhao, Michelle P. Lapak, Mukunth Saravanan, Ryan A. Hackler, Yi-Yu Wang, Long Qi, Massimiliano Delferro, Tao Li, Byeongdu Lee, Baron Peters, Kenneth R. Poeppelmeier, Salai C. Ammal, Clifford R. Bowers, Frédéric A. Perras, Andreas Heyden, Aaron D. Sadow, and Wenyu Huang, and published in the Nature Catalysis.
The research was conducted by the Institute for Cooperative Upcycling of Plastics (iCOUP), led by Ames National Laboratory. iCOUP is an Energy Frontier Research Center consisting of scientists from Ames National Laboratory, Argonne National Laboratory, UC Santa Barbara, University of South Carolina, Cornell University, Northwestern University, and the University of Illinois Urbana-Champaign.
Ames National Laboratory is a U.S. Department of Energy Office of Science National Laboratory operated by Iowa State University. Ames Laboratory creates innovative materials, technologies, and energy solutions. We use our expertise, unique capabilities, and interdisciplinary collaborations to solve global problems.
Ames Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit https://energy.gov/science.
Published In: Institute for Cooperative Upcycling of Plastics