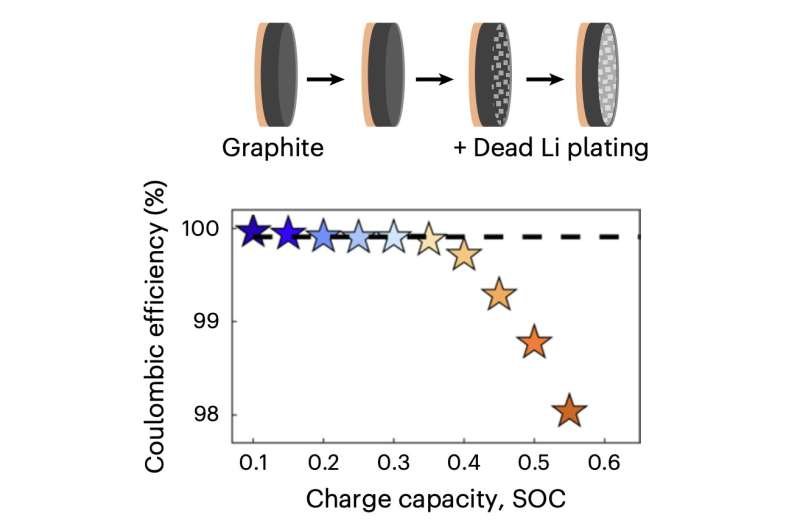
The main reason for this is that during fast charging, lithium plating could form on the batteries' graphite anode, which could pose safety risks. In fact, lithium plating reactions on graphite anodes, which can also occur at low temperatures, during overcharging or following battery malfunctions, can lead to the formation of non-cyclable lithium metal and salts, which could ignite causing fires or battery explosions.
Researchers at University of California, Berkeley and the Lawrence Berkeley National Laboratory recently carried out a study investigating potential ways to reduce these risks and enable the creation of safe fast-charging LiBs. Their paper, published in Nature Energy, outlines a series of simple techniques for quantifying irreversible Li plating on the graphite anodes inside LiBs.
"We demonstrate the power of simple, accessible and high-throughput cycling techniques to quantify irreversible Li plating spanning data from over 200 cells," Zachary M. Konz, Brandan M. Wirtz and their colleagues wrote in their paper. "We first observe the effects of energy density, charge rate, temperature and state of charge on lithium plating, use the results to refine a mature physics-based electrochemical model and provide an interpretable empirical equation for predicting the plating onset state of charge. We then explore the reversibility of lithium plating and its connection to electrolyte design for preventing irreversible Li accumulation."
The experiments carried out by Konz and his colleagues highlighted the value of their proposed techniques for quantifying lithium plating in Li/Graphite and graphite/NMC battery cells. Using these techniques, they were able to gather valuable insight about the plating processes in these types of batteries, as well as improvements that could improve their safety and potentially enable fast-charging.
"We design a method to quantify in situ Li plating for commercially relevant graphite|LiNi0.5Mn0.3Co0.2O2 (NMC) cells and compare with results from the experimentally convenient Li|graphite configuration," Konz and his colleagues explained in their paper. "The hypotheses and abundant data herein were generated primarily with equipment universal to the battery researcher, encouraging further development of innovative testing methods and data processing that enable rapid battery engineering."
The recent work by this team of researchers contributes to ongoing efforts aimed at further improving the safety of LiBs and speeding up their charging times. So far, detecting lithium plating on these batteries' anodes was deemed very difficult, which hindered the creation and large-scale deployment of fast-charging LiBs.
The lithium plating detection techniques proposed by Konz and his colleagues could soon inform further research aimed at devising safer fast-charging LiB designs. In addition, they could help to better understand the factors and processes that promote plating, which could in turn guide research works exploring alternative battery designs and chemistries.