Researchers from the University of Twente in The Netherlands have developed a new high-entropy perovskite oxide (HEO) as a high-activity electrocatalyst for the oxygen evolution reaction (OER)—the key kinetically limiting half-reaction in several electrochemical energy conversion technologies, including green hydrogen generation.
In an open-access study in the journal ACS Nano, the researchers report that the composite (LaCr0.2Mn0.2Fe0.2Co0.2Ni0.2O3-δ) outperforms its parent compounds with 17 to 680 times higher currents at a fixed overpotential. The composite, which consists of several earth-abundant elements, could potentially be used for efficient hydrogen generation without rare and precious metals such as platinum.
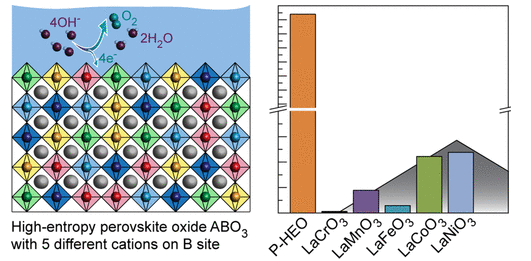
Currently, the most efficient electrolyzers contain platinum and iridium, which are needed for the electrodes on which the hydrogen and oxygen gas are produced from water. However, platinum and especially iridium are too rare. That’s why we’re constantly looking for electrode materials made from more abundant resources which also can be used as efficient and stable electrocatalysts.
—Chris Baeumer, co-corresponding author
We expected that the stability compared to traditional composites would be enhanced, but when we started testing it soon turned out that the activity was much higher too. In collaboration with our partners from Karlsruhe (Germany) and Berkeley (USA), we found that the individual transition metals may ‘help’ each other to make the combined material better than the sum of its parts in a so-called synergy effect.
—Chris Baeumer