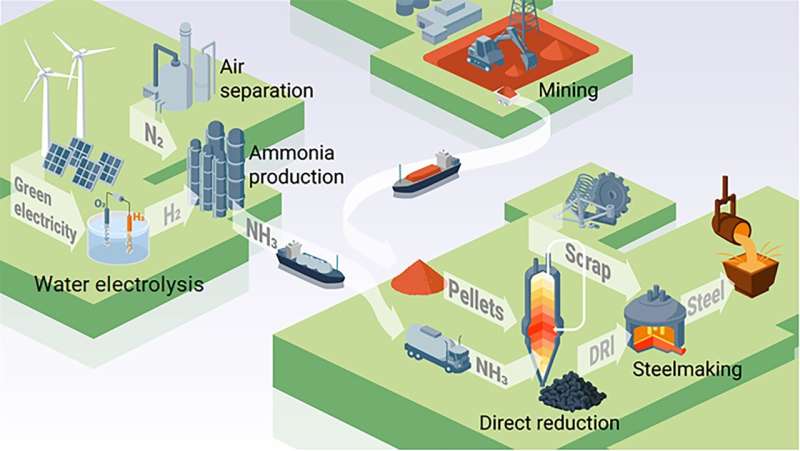
When it comes to sustainability and green steel, everybody talks about hydrogen. But current means of storing and transporting hydrogen require high pressures and low temperatures, which are both energetically and economically costly. Ammonia is known to be a good hydrogen carrier. Yan Ma and colleagues show that ammonia can not only be used to carry hydrogen but also for the direct reduction of iron, which makes ammonia a viable candidate to overcome the shortcomings of hydrogen.
Max Planck materials scientists use ammonia for sustainable iron- and steelmaking. They have published their latest findings in the journal Advanced Science
Steel production is currently the biggest single cause of global warming, responsible for about 7% of global CO2 emissions. To cut these emissions, scientists in the industry are intensively investigating hydrogen-based ironmaking approaches as sustainable pathways to replace carbon reductants.
While the hydrogen-based direct reduction of iron ore is promising, researchers are facing one major challenge: to make the whole steel making process climate friendly, the used energy and hydrogen themselves should be produced in sustainable ways. But markets are lacking enough green hydrogen and current means of storing and transporting hydrogen request high pressures and low temperatures, which are both energetically and economically costly.
Researchers of the Max-Planck-Institut für Eisenforschung (MPIE) tackled this challenge by using ammonia as a hydrogen carrier and as a reductant for iron. They compared the iron and steel produced with ammonia-based direct reduction with hydrogen-based direct reduction, analyzed the characteristics of the novel process and costs and published their results in the journal Advanced Science.
Ammonia as direct reductant brings several advantages
An increasing amount of hydrogen is needed worldwide, but the storage and transport of hydrogen is tricky: it has to be either stored at very low temperatures or high pressures due to its low volumetric energy density. These conditions cost 30% of the embodied chemical energy hydrogen delivers. By contrast, ammonia is already traded worldwide with established logistics and is known to be an excellent hydrogen carrier with low liquefaction costs.
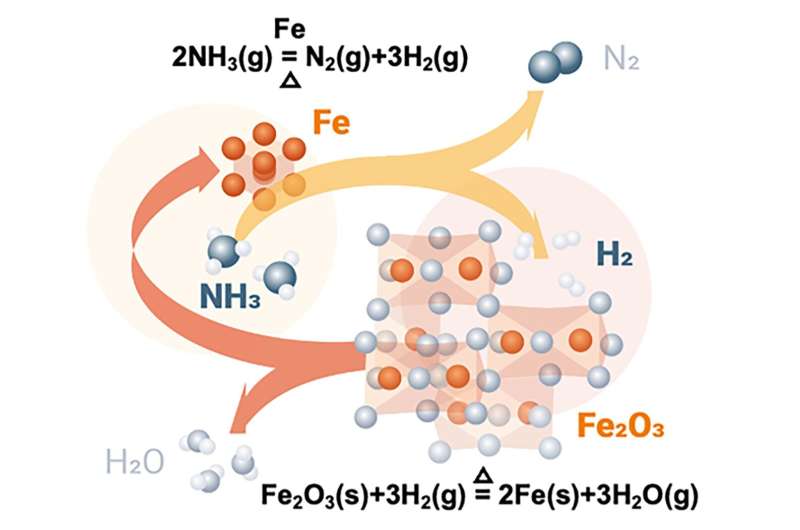
"Our aim was to study whether ammonia can be directly used to reduce iron ores without cracking it into hydrogen and nitrogen. Avoiding this cracking process can reduce the overall costs by 18%. Moreover, we analyzed how ammonia as reduction agent affects the properties of the reduced iron," explains Dr. Yan Ma, group leader at MPIE and first author of the publication.
The scientists introduced ammonia in a laboratory-scale reactor where iron ores are reduced to so-called sponge iron. During this process, a thermogravimetry coupled with a mass spectrometry measured the weight and gas composition showing the reduction degree and the onset of ammonia decomposition. "The ammonia-based direct reduction proceeds through an autocatalytic reaction. We compared its kinetics with the hydrogen-based direct reduction. Both have similar characteristics and yield the same metallization degree. In contrast to hydrogen-based reduction, nitrides form during cooling in ammonia, which could protect the sponge iron from corrosion and make it easier to handle," explains Ma.
The nitride phase can be completely dissolved and removed during the subsequent melting process, which is necessary for downstream processing. Moreover, the other product of ammonia decomposition, nitrogen, can act as a heat carrier in a shaft furnace to maintain the reaction temperature and enhance the efficiency for the reduction of iron ores.
Outlook: Synthesizing green ammonia and tuning the iron reduction process
The ammonia-based direct reduction connects two of the most CO2 intensive industries, steel and ammonia production, and paves the way to a sustainable transition together. Moreover, by using ammonia the logistic and energetic disadvantages of hydrogen are overcome and already existing furnace technologies, namely shaft and electric arc furnaces, can be used with only slight modification.
In the next step, the Max Planck team will test different process parameters like temperature or gas mixture to speed up the ammonia-based reduction process for a wide industrial application.