Now, researchers at Michigan Technological University have demonstrated a carbonate-superstructured solid fuel cell (CSSFC) in which in situ generation of superstructured carbonate in the porous samarium-doped ceria layer creates a unique electrolyte with ultrahigh ionic conductivity of 0.17 S⋅cm−1 at 550 °C. The CSSFC shows enhanced power density with hydrocarbon fuels at lower operating temperatures. An open-access paper on the work appears in Proceedings of the National Academy of Sciences (PNAS).
… low-temperature SOFCs (LT-SOFCs) with hydrocarbon fuels suffer from polarization losses caused by temperature drop and carbon deposition (coking). This happens because 1) the hydrocarbon oxidation kinetics are extremely sluggish at lower temperatures due to the strong C–H bonds and 2) carbon deposition deactivates electrodes by covering catalytic sites.
… one of the key strategies to improve hydrocarbon oxidation and reduce coking for LT-SOFCs is to increase the oxygen ionic conductivity of electrolytes. … There exist two conventional strategies to enhance the oxygen ionic conductivity of electrolytes in LT-SOFCs, namely, reducing electrolyte thickness and developing fast ionic conductors. The ultrathin electrolyte film requires advanced techniques and inevitably increases fabrication cost and complexity. Although bismuth oxides exhibited impressive oxygen ionic conductivity due to their rich oxygen vacancies, their poor stability under SOFC operation conditions would hinder their applications. Therefore, other strategies are required to develop efficient ionic conductors.
—Su et al.
The team hypothesized that a continuous interface between molten carbonate and solid ionic conductor could constitute a fast transfer channel for oxygen ions—i.e., such a carbonate superstructure on solid ionic conductor would be an oxygen ionic superconductor.
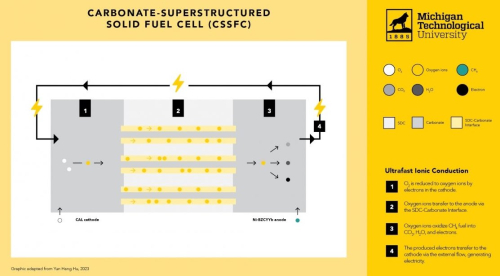
To test this hypothesis, we fabricated a device by integrating a LiNi0.8Co0.15Al0.05O2 (NCAL) cathode, a porous Ce0.8Sm0.2O1.9 (SDC) electrolyte, and a Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3–δ (BZCYYb) anode using a one-step dry-pressing procedure without high-temperature sintering in this work. The electrodes and the electrolyte remain porous and nanocrystalline structures in the system. Then, molten carbonate in the porous NCAL and SDC layers is in situ generated at cell operation conditions, creating the carbonate-superstructured fuel cell (CSSFC).
Furthermore, the CSSFC exhibited ultrahigh ionic conductivity of 0.17 S⋅cm−1 at 550°C, leading to an unprecedented high open-circuit voltage (OCV) and a very high peak power density (PPD) as well as excellent coking resistance with dry methane fuel at 550°C.
—Su et al.
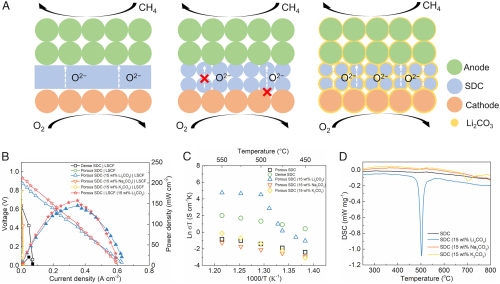
(A) Schematic of conventional SOFC, porous SOFC, and the CSSFC. (B) The I-V-P performance of different fuel cell configurations with Ni-BZCYYb as anodes operated on CH4 at 550 °C. (C) The temperature-dependent Arrhenius plot of oxygen ionic conductivities of different electrolytes with or without carbonate modification. (D) DSC plots of different electrolytes in Ar atmosphere. Su et al.
Corresponding author Yun Hang Hu estimates that CSSFC fuel efficiency could reach 60%. By comparison, the average fuel efficiency of a combustion engine ranges between 35% and 30%. The CSSFC’s higher fuel efficiency could lead to lower carbon dioxide emissions in vehicles.