Researchers at MIT say they have created a new material that will pave the way to faster charging batteries.
Increasing demand for improved electrochemical energy storage devices—that is, batteries—stems from a broad spectrum of technology. That includes electric vehicles, municipal power backup systems that require uninterrupted power during temporary outages, and various other applications in the agricultural, biomedical and defense sectors.
More efficient batteries will contribute to increasing demands for a greener, sustainable future.
But state-of-the-art battery technology suffer from a few drawbacks. One is the length of time required to recharge batteries. Another challenge, as noted in a report by the MIT researchers in the journal Joule, is designing batteries that combine high charge capacity with "long cycling life of capacitors."
Lithium-ion cells (LIC), they explain, are the leading electrochemical energy storage devices in use today. They boast high charge capacity but fall short when it comes to recharging times.
As an alternative, designs that incorporated plentiful, inexpensive organic materials were proposed. But they were found to suffer from diminished electrical conductivity. Other approaches that utilized modular designs that combined LICs and capacitors within specific devices proved overly complex and costly.
Those problems, according to MIT graduate student Tianyang Chen, author of the Joule report, "create a strong incentive to develop electrode materials that combine the high charge capacity of LICs with the fast charging and long cycling life of capacitors."
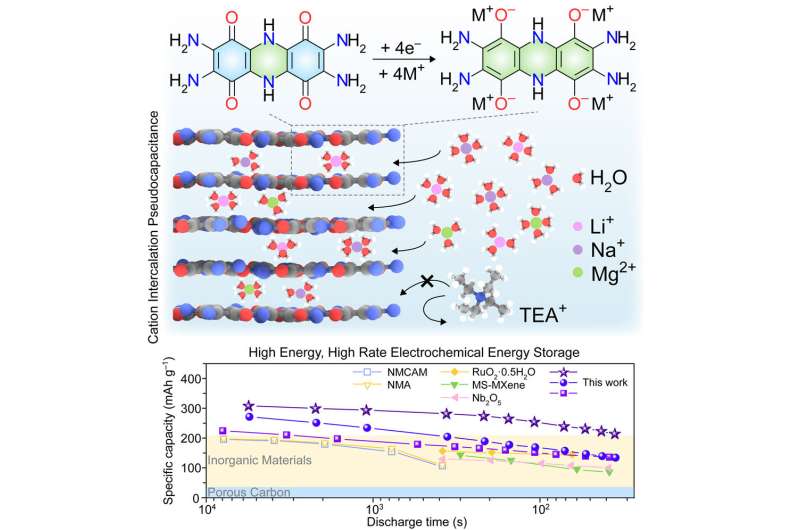
Chen and his team came up with a combination of organic materials that can be used in battery cathodes, where lithium is stored in discharged batteries.
Attempts by others to use organic materials failed because they dissolved in battery electrolytes.
"Although strategies such as polymerization and compositing organic molecules with insoluble admixers can limit electrode dissolution," Chen said, "designing organic materials that are themselves insoluble but still allow swift charge transport and storage is difficult."
But the materials created in MIT laboratories remain stable, Chen said.
"We report bis-tetraaminobenzoquinone and its polymeric analog poly(bis-tetraaminobenzoquinone) as pseudocapacitive organic materials" for electrical energy storage devices, he said. They "exhibit high charge storage capacities at high charge-discharge rates."
The organic materials are capable of storing 310 milliampere hours of charge, which is roughly double the capacity of the current crop of lithium-ion battery cathodes.
The charge time for the battery with those materials is a speedy 33 seconds.
Relying on abundant magnesium or sodium ions, rather than less-readily available lithium, will produce optimal results at lower cost, he said.