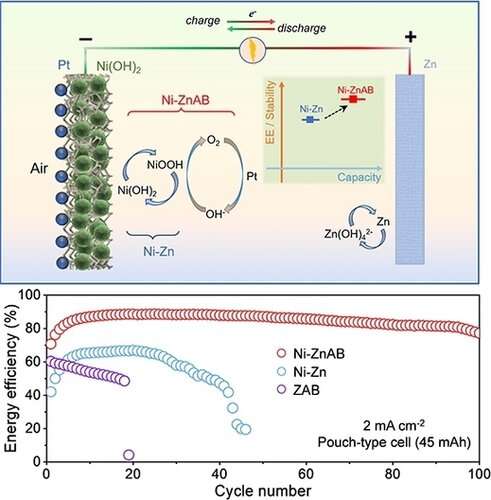
Nickel-zinc (Ni-Zn) batteries are a promising technology due to their high output voltage, high theoretical specific energy, high safety and low cost. However, rechargeable alkaline Ni-Zn batteries are challenging, because the cathodic side reaction of oxygen evolution results in low energy efficiency and poor stability.
Recently, a research group led by Prof. Yang Weishen and Dr. Zhu Kaiyue from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences proposed to construct an air-breathing cathode using side oxygen evolution reaction (OER) in Ni-Zn batteries by coupling electrocatalysts for oxygen reduction reactions (ORR) in the cathode.
This novel battery (Ni-ZnAB) exhibited an ultra-long lifespan and ultra-high energy efficiency (85%), superior to Ni-Zn battery and Zn-air battery.
This study was published in Angewandte Chemie International Edition on March 27.
The inevitable OER in the charging process of Ni-Zn reduces energy efficiency and Coulombic efficiency, leading to poor energy storage and release capabilities. Although the OER in the cathode can be partially suppressed by controlling the charging voltage (but at the expense of capacity) and using electrolyte additives, these strategies are unfortunately insufficient to completely solve the OER issue in the Ni-Zn system.
In this work, the researchers proposed leveraging the OER in Ni-Zn batteries by coupling electrocatalysts for ORR in the cathode. This approach would enable the oxygen generated during charging via the OER to be utilized during discharge, similar to the air-breathing mechanism.
In these Ni-Zn batteries with air-breathing cathodes (named as Ni-ZnAB batteries), the OER in the cathode was no longer an undesirable side reaction during the charging process. Furthermore, the Coulombic efficiency loss from nickel hydroxide could be compensated by the ORR. Compared to conventional Ni-Zn batteries, the novel Ni-ZnAB batteries exhibited significantly improved cycling stability and energy efficiency.
Due to the stabilizing effects on the electrolyte and electrode, the pouch-type Ni-ZnAB battery exhibited an excellent cycling performance of 100 hours with a capacity of 45 mAh and an average energy efficiency of 85.1%, indicating the potential of the Ni-ZnAB battery for practical applications. To further improve the cycling stability, a mold-type Ni-ZnAB battery with a rich electrolyte was designed, which delivered an ultrahigh stability of 500 cycles with an energy efficiency higher than 80%, showing significant improvement compared to Ni-Zn.
"Our results highlight the importance of incorporating air-breathing cathode in Ni-Zn cells to improve their stability and energy efficiency, and showcase the potential of Ni-ZnAB batteries as a valuable guide for designing highly stable Ni-Zn batteries," said Prof. Yang.