Adiponitrile is an organic compound that is an important precursor to the polymer nylon 66.
The surface of the electrolyte possesses a liquid nano-layer of Adpn that links grains for a facile ionic conduction without high pressure/temperature treatments. The material can quickly self-heal if fractured and provides liquid-like conduction paths via the grain boundaries.
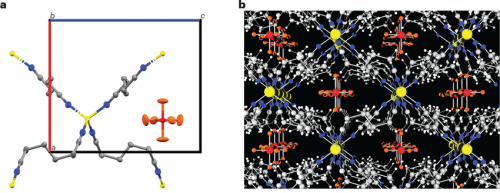
Crystal structure of soft solid co-crystalline (Adpn)2LiPF6 electrolyte a, Representation of the basic structural unit of (Adpn)2LiPF6 showing tetra-coordinated Li⁺ ions with four Adpn molecules, each shared with a second symmetry-equivalent Li atom; PF6⁻ anions occupy the available interstitial pocket in the crystal structure. Grey, C; yellow, Li; blue, N; red, P; orange, F.; colored box shows the dimensions of unit cell; red, a-crystallographic direction; blue, c-crystallographic direction. b, Packing diagram of (Adpn)2LiPF6 showing the channels of Li⁺ ions in the low-affinity matrix in the crystal structure. Prakash et al.
A substantially high ion conductivity (~10−4 S cm–1) and lithium-ion transference number (0.54) are obtained due to weak interactions between ‘hard’ (charge dense) Li+ ions and the ‘soft’ (electronically polarizable) –C≡N group of Adpn.
Molecular simulations predict that Li+ ions migrate at the co-crystal grain boundaries with a (preferentially) lower activation energy Ea and within the interstitial regions between the co-crystals with higher Ea values, where the bulk conductivity is a smaller but extant contribution.
These co-crystals establish a special concept of crystal design to increase the thermal stability of LiPF6 by separating ions in the Adpn solvent matrix, and also exhibit a unique mechanism of ion conduction via low-resistance grain boundaries, which contrasts with ceramics or gel electrolytes.