A research team led by Professor Yong-Tae Kim (Department of Material Science and Engineering and Graduate Institute of Ferrous and Energy Materials Technology) and Ph.D. candidate Sang-Hoon You (Department of Material Science and Engineering) at POSTECH has developed a selective catalyst that curbs corrosion in fuel cells used for hydrogen-powered automobiles.
By tailoring the hydrogen oxidation reaction to match the concentration of hydrogen in the fuel cell, the team was able to hinder the corrosion of the fuel cells. The research was published in ACS Energy Letters.
Fuel cells are susceptible to numerous factors that deteriorate their durability. One of them is degradation, especially in the cathode catalyst, which is routinely exposed to start-up and shut-down events in automobiles. In particular, fuel cells designed for automotives experience recurring cycles of start-up and shut-down by nature.
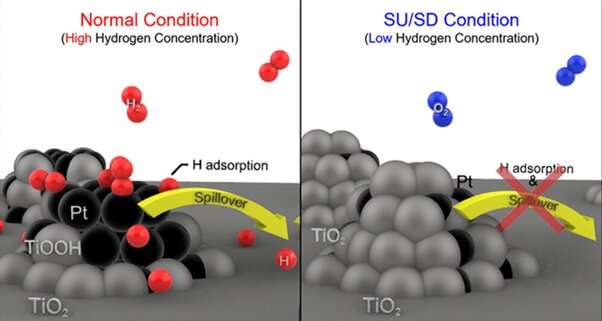
During normal vehicle operation, fuel cells are supplied with a consistent supply of hydrogen with high concentration, but the concentration of hydrogen temporarily declines when the car is turned off or started. Consequently, when external air mixes with hydrogen within the fuel cells, an unintended oxygen reduction reaction in the anode is triggered, leading to sudden potential jumps and carbon corrosion in the cathode.
The research team has engineered a catalyst (Pt/TiO2), comprising platinum (Pt) deposited onto a titanium dioxide (TiO2), that efficiently halts corrosion in fuel cells employed in hydrogen-powered automobiles. The performance of this electrocatalyst comes from the robust interaction between titanium dioxide and platinum, and the ability of hydrogen spillover to modify the surface conductivity of the material in response to the hydrogen concentration in its vicinity.
When a vehicle suddenly stops or starts, the concentration of hydrogen within the fuel decreases correspondingly. As a consequence of this reduction in hydrogen concentration, there is an expansion of titanium dioxide onto platinum, which results in platinum being buried beneath the catalyst's surface.
This burying of the platinum, caused by expansion of titanium dioxide, ultimately transforms the catalyst into an insulator due to the low conductivity of titanium dioxide. This insulating effect hinders the catalyst's ability to conduct electricity, thus preventing an unwanted reduction of oxygen that could cause sudden potential jumps in the cathode.
Conversely, during a standard vehicle operation, the concentration of hydrogen within the car remains high. Under such high hydrogen concentration conditions, the highly conductive platinum is exposed on the catalyst's surface, and titanium dioxide reduction occurs, which promotes hydrogen mobility on the catalyst's surface. This phenomenon, termed hydrogen spillover, enhances current flow and increases hydrogen oxidation reaction.
The research team also performed a simulation test to compare the newly developed catalyst and conventional catalysts. The test results demonstrated that fuel cells using Pt/TiO2 catalyst exhibited three-times-higher durability, relative to traditional fuel cells. This indicates the team successfully increased the durability of fuel cells through the use of a selective oxygen reduction reaction and a hydrogen oxidation reaction based on the hydrogen concentration.
If this research can contribute to overcoming the existing durability challenges confronting fuel cells for hydrogen-powered vehicles, then it could potentially elevate the standing of Korean hydrogen-fueled automobiles in the next-generation mobility industry.







