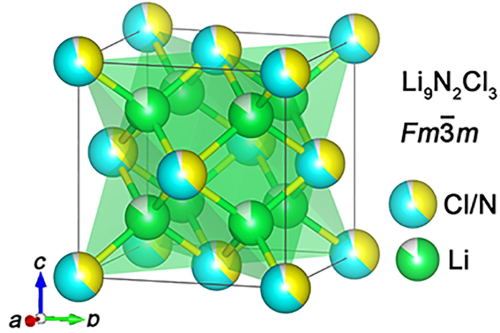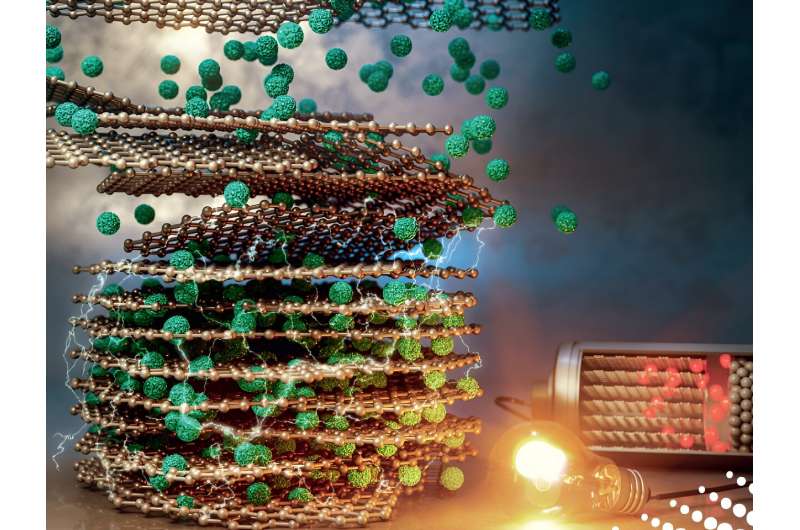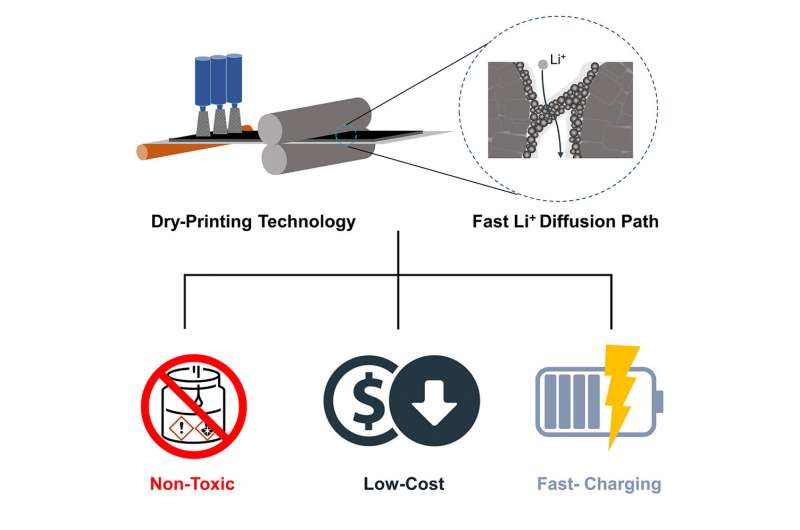
Wang, who is the WPI William B. Smith Dean's Professor in the Department of Mechanical and Materials Engineering, said the new process could be scaled up and reduce electrode manufacturing costs by up to 15 percent, while also producing electrodes that can charge faster than conventionally produced electrodes.
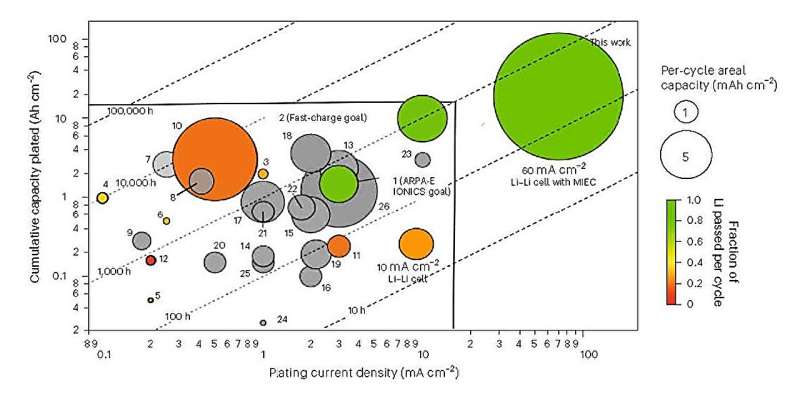
"Current lithium-ion batteries charge too slowly, and manufacturers typically use flammable, toxic, and expensive solvents that increase the time and cost of production," Wang said. "Our solvent-free manufacturing process addresses those disadvantages by producing electrodes that charge to 78 percent of capacity in 20 minutes, all without the need for solvents, slurries, and long production times."
Commercial lithium-ion battery electrodes are typically made by mixing active materials, conductive additives, polymers, and organic solvents to create a slurry that is pasted onto a metal substrate, dried in an oven, and cut into pieces for use in batteries. The solvents are recovered through distillation.
The researchers' process, in contrast, involved mixing together dry powders that were electrically charged so they would adhere when sprayed onto a metal substrate. The dry-coated electrodes were then heated and compressed with rollers. Skipping the conventional drying and solvent-recovery process cut battery manufacturing energy use by an estimated 47 percent, the researchers reported.
Wang has long been focused on improving lithium-ion batteries and reducing the waste they create. He co-founded Ascend Elements, a company that is developing battery recycling technologies.