Lithium metal batteries are promising battery solutions that use metallic lithium as an anode. Instead of containing electrode materials that store lithium ions, like commonly employed lithium-ion batteries (LiBs), they use a single layer of lithium near the anode, which can greatly reduce their size and weight.
While these batteries could outperform LiBs in terms of capacity and energy density, to function reliably, the layer between their Li metal anode and electrolyte should be robust and stable over time. Stabilizing this layer, known as the solid electrolyte interphase (SEI), has so far proven to be challenging.
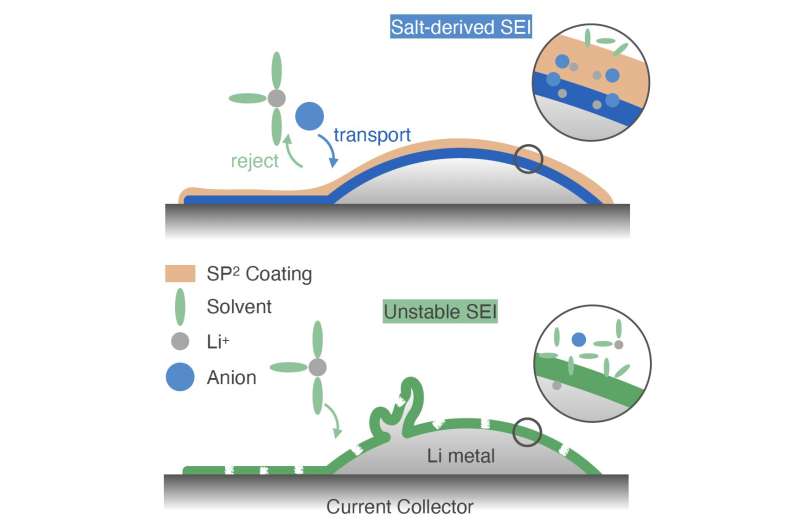
Researchers at Stanford University have recently introduced a new strategy that could produce a more stable SEI in lithium metal batteries, which would in turn improve their overall cycling performance. Their proposed method, introduced in a paper in Nature Energy, entails coating electrodes in the battery cell with a salt-philic and solvent-phobic polymer.
"The origin of Li metal battery capacity fading is the reaction between Li and the electrolyte," Rachel Z Huang, one of the researchers who carried out the study, told Tech Xplore. "Specifically, Li reacts with the salt and solvents to form an SEI layer. Recent research has uncovered that a SEI derived from Li reacting with salt (salt-derived SEI) is more stable than a solvent-derived SEI, and it is correlated with a longer cell cycle life."
To induce a salt-derived SEI formation in lithium metal batteries, Huang and her colleagues decided to cover electrodes using a carefully engineered polymer coating. The polymer they created consists of a polysiloxane backbone with pyridinium bis(trifluoromethylsulfonyl)imide (PyTFSI) and perfluorinated side chains.
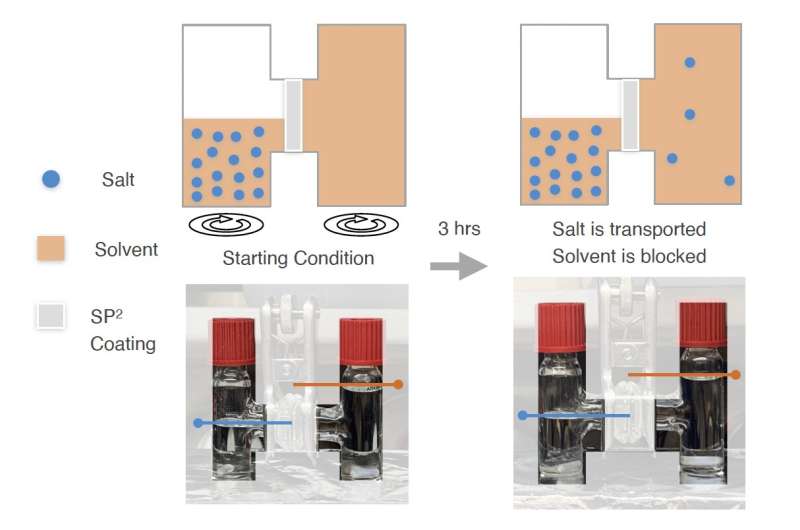
"Our aim was for the polymer to simultaneously increase the presence of salt and decrease the presence of solvent at the interface, thereby driving salt-derived SEI formation," Huang explained. "We have designed a siloxane-based polymer with tethered salt-philic and solvent-phobic (SP2) side chains. This polymer exhibits selective transport of salt over solvent in an H-Cell experiment and has increased the content of salt-derived SEI with three major types of electrolytes."
The formulation of the polymer coating used by Huang and her colleagues was identified after exploring several possible side chain chemistries. In their initial analyses, it was found to be an optimal solution both in terms of its electrochemical performance and how it interacts with salt and solvent.
The researchers evaluated their strategy in a series of experiments and found that it could regulate the SEI inside lithium metal battery cells, prolonging their cycle life. They also tested their polymer-coating approach in combination with different electrolytes, including ether (lithium bis(trifluoromethanesulfonyl)imide in 1,3-dioxolane/1,2-dimethoxyethane with 1wt% LiNO3), carbonate (lithium hexafluorophosphate in ethylene carbonate/diethyl carbonate with 10% fluoroethylene carbonate), and fluorinated 1,4-dimethoxylbutane electrolyte (FDMB).
"In this work, we utilized physics to tune chemistry, achieving one of the state-of-the-art cycling performances for Li metal batteries," Huang said. "The key features of our approach are its flexibility in design and versatility in application. Regarding design flexibility, we demonstrated that our interface design can be modulated, which means that this property can be achieved using different polymer side chains and/or polymer backbones. Furthermore, this modulated design concept can be extrapolated to composites, allowing for even greater design possibilities."
So far, the researchers have shown that their polymer coating is compatible with three major types of electrons contained in lithium metal batteries. However, when more promising electrolytes are uncovered, the set of electrolytes that respond well to their method could be extended further.
As it appears to generalize well across cells with various compositions, this promising design strategy could soon be employed by other teams worldwide to improve the performance and stability of lithium metal batteries. Huang and her colleagues are also planning to carry out additional studies assessing their method's benefits and applicability.
"Other works that aim to increase interfacial stability typically focus on a specific electrolyte, whereas our approach offers broader applicability and addresses multiple electrolyte systems," Huang added. "We plan to explore the materials design space a bit more to find out what other compositions can also achieve these salt-phobic and solvent-philic properties, as well as explore the potential of this design paired with novel electrolyte formulations."