Dominant battery technologies using flammable, toxic, unsustainable, and expensive energy sources are a major contributor to climate change. Switching from fossil fuels to cleaner and environmentally friendly energy sources is thus crucial to curtail the impacts of climate change. This transition can be supported by improving the efficiency of energy storage systems for safer and stable operations, sustainability, high energy/ power density.
Research on this front has focused on molecular engineering approaches to the development of aqueous-based redox-enhanced electrochemical capacitors (redox ECs). Redox ECs are a type of advanced hybrid electric double-layer capacitors that use redox-active molecules at the electrode-electrolyte interface to increase the energy density.
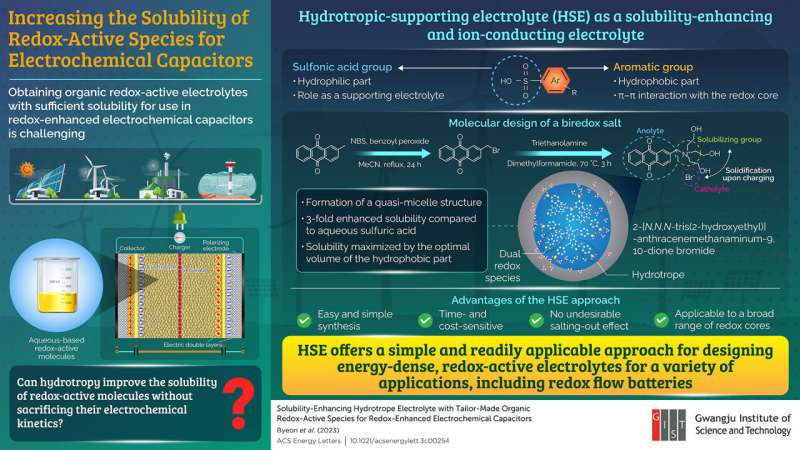
Owing to the use of organic redox-active electrolytes, they are known to provide a cost merit, use of earth-abundant elements, and structural tunability. However, a major challenge in their development is the lack of sufficient solubility of these species in aqueous systems, which results in a low energy density. Furthermore, previous attempts at improving their solubility have proven to be time-consuming and cost extensive.
Now, researchers from Korea have used hydrotropic-supporting electrolyte (HSE) as an approach to enhancing the solubility of the organic redox-active species. The study, led by Assistant Professor Seung Joon Yoo and Professor Sukwon Hong from Gwangju Institute of Science and Technology in Korea, was published in ACS Energy Letters.
The researchers used the process of hydrotropy, wherein a class of amphiphilic molecules are used. In this unique solubilization phenomenon, the volume of the hydrophobic component is relatively small compared to that of the surfactant, thus allowing an increase in the solubility of the sparingly soluble solute multifold. The researchers tested a range of quinones as a model species owing to their utility as a redox-active additive and an acceptable electrochemical stability.
The researchers found that using the HSE (p-toluene sulfonic acid (p-TsOH), 2-naphthalenesulfonic acid (2-NpOH), and anthraquinone-2-sulfonic acid (AQS)) improved the solubility of hydroquinone (HQ) without any chemical functionalization. Importantly, they demonstrated that an increase in the solubility is proportional to the concentration of the respective HSEs.
Moreover, they designed a biredox salt, 2-[N,N,N-tris(2-hydroxyethyl)] anthracenemethanaminum-9,10-dione bromide (AQM-Br), which could participate in Faradaic reactions at both the positive and negative electrodes, and tested it in the HSE system in a concentration-dependent manner. Dr. Yoo highlights, "The solubility of HQ in HSE was increased 7-fold, and a designer multifunctional dual-redox species (AQM-Br) was synthesized, the solubility of which was significantly enhanced from being barely soluble to >1 M by optimizing the HSE."
Furthermore, the researchers also attempted to understand the action of solubilization for both the HQ and AQM-Br electrolyte. Using the intermolecular nuclear Overhauser effect and dynamic light scattering analyses, they found that the hydrotrope solubilization for HQ/HSE was achieved through the co-solubilizer mechanism, whereas for AQM-Br/HSE, it was due to the formation of quasi-micelle nanostructures.
Explaining the potential implications of the study, Prof. Yoo concludes, "Our simple approach can be readily extended to a different class of redox species and [be] applicable to a wide variety of applications including redox flow batteries. In addition, our study provides a guideline for the design of energy-dense redox-active electrolytes and an optimal selection of HSE and redox-active electrolyte pairs."