Scientists in Australia and China are hoping to make the world's first safe and efficient non-toxic aqueous aluminum radical battery.
Teams from Flinders University in South Australia and Zhejiang Sci-Tech University in China have reported the first stage of developing these novel batteries in an article entitled, Lewis Acid-Induced Reversible Disproportionation of TEMPO Enables Aqueous Aluminum Radical Batteries, published by the Journal of the American Chemical Society.
Most batteries contain hazardous materials and can pollute the environment when disposed of in landfills or when thrown out elsewhere. Materials like lead, cadmium and mercury can poison people and animals and contaminate soils and water, and they stay in the environment for a long time.
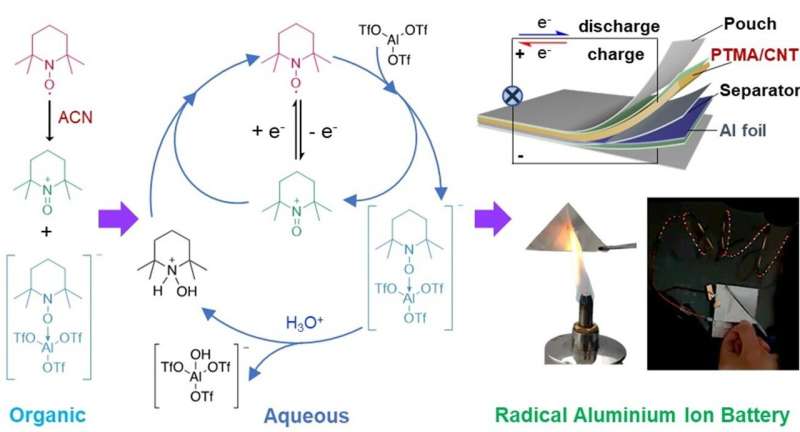
Dr. Kai Zhang, from Zhejiang Sci-Tech University, and Associate Professor Zhongfan Jia's research lab at Flinders University collaborated on the (electro)chemistry of stable radicals in the most-used Lewis acid electrolyte (Al(OTf)3 and battery test.
The team developed the first design of aluminum radical batteries which use water-based electrolytes that are fire-retardant and air-stable, delivering a stable voltage output of 1.25 V and a capacity of 110 mAh g–1 over 800 cycles with only 0.028% loss per cycle.
Professor Zhongfan Jia, from Flinders University's College of Science and Engineering, hopes to use biodegradable materials for development of the soft-pack batteries in the future to make the product safe and sustainable.
Multivalent metal ion batteries, including Al3+, Zn2+ or Mg2+, use abundant elements of the Earth's crust and provide much higher energy density than lithium-ion batteries (LIBs), says Professor Jia.
"In particular, aluminum-ion batteries (AIBs) attract great attention because aluminum is the third most abundant element (8.1%), which makes AIBs potentially a sustainable and low-cost energy storage system."
However, one of the major challenges for current AIBs is the slow movement of Al3+ ion complexes, which lead to AIBs with low cathode efficiency. Organic conjugated polymers are emerging cathodes for AIBs to address the ion transport issue but their battery voltage output performance remains poor.
Stable radicals are a class of organic electroactive molecules that have been widely used in different organic battery systems. The first of this kind was commercialized by NEC in 2012.
The Jia Lab at Flinders University has previously developed radical materials for organic hybrid LIBs, sodium-ion batteries, and all-organic batteries. These radical materials have never been applied in AIBs due to lacking understanding of their (electro)chemical reaction in electrolytes.