A team of researchers at City University of Hong Kong, Northwestern University, and other institutes in the United States has been trying to devise strategies and solutions that could improve the performance of LiBs, extending their life and increasing their energy capacity. In a recent paper published in Nature Energy, the team introduced a new cathode that could increase the capacity of LiBs, by tackling a well-documented limitation of existing cathodes.
"Imagine a world where your phone's battery lasts much longer and electric cars can go further on a single charge," Prof. Qi Liu, one of the researchers who carried out the study, told TechXplore. "That's the dream we're working towards. By improving LiBs, the power sources of modern electric devices and electric vehicles. Our recent study focuses on a specific type of material called Lithium- and Manganese-rich (LMR) layered cathodes, which have the potential to store much more energy than the current commercial cathodes."
LMR layered cathodes are known to be susceptible to a phenomenon known as 'voltage decay', which entails a rapid deterioration of cathodes and a loss of voltage in the battery. This crucial and extensively investigated issue significantly limits the performance of LiBs, limiting their overall potential as a battery solution.
"We were inspired by the challenges faced by researchers worldwide who were trying to tackle this voltage decay issue," Prof. Liu explained. "Some brilliant minds had already proposed ideas, like coating the cathode material or introducing new elements to stabilize the structure. But even with these efforts, the problem remained unsolved."
In recent times, there has been a surge of interest in a class of LMR materials characterized by a unique O2-stacked structure. Notably, these materials exhibit lower voltage decay compared to conventional O3-type LMRs. The distinctive O2 stacking also offers an opportunity to finely adjust the local structure of the inherently unstable honeycomb lattice.
Drawing inspiration from these findings, Prof. Liu and his colleagues attempted to construct a new O2-type based LMR cathodes. The researchers' primary goal was to stabilize the characteristic honeycomb-shaped structure of LMR materials.
"We introduced transition metal (TM) ions into the lithium layers above or below the honeycomb structure," explained by Prof. Liu. "Our goal was to significantly enhance the stability of the honeycomb structure, effectively mitigating the voltage decay issue during cycling and enabling the practical application of LMR cathode materials in high-energy-density LIBs."
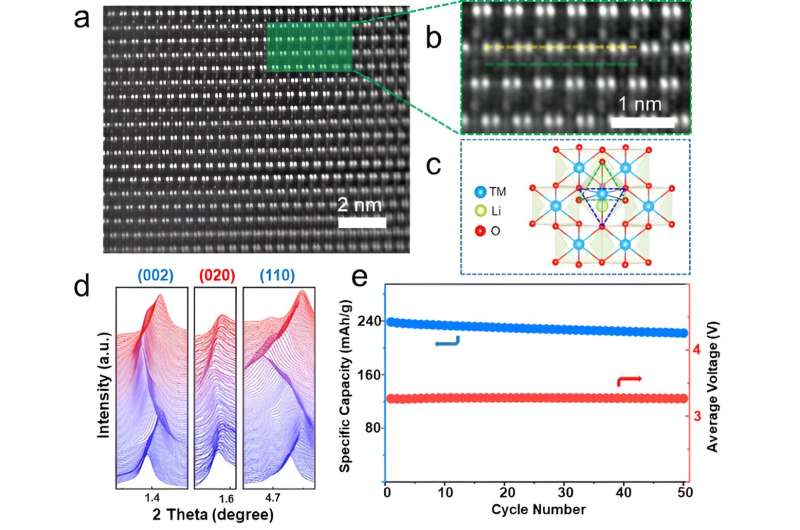
To create a LMR material with a TM-pinned honeycomb, the researchers used an ion-exchange technique (i.e., a system to efficiently remove or dissolve ions.) This technique allowed them to transform a P2-type Na0.6Li0.2(Mn0.6-xNix)O2 material into their desired O2-type LMR cathode.
"The unique characteristics of the material we created is the incorporation of TM ions strategically located above or below the honeycomb-like structure, acting as a cap to stabilize the fragile framework," Prof. Liu said. "The advantage of our LMR cathode is the significantly lower voltage decay during battery use compared to traditional cathodes."
In the initial evaluations, the Li-rich cathode created by Prof. Liu and his colleagues performed remarkably well, suggesting that it could extend the life and enhance the performance of LIBs. This could translate to electronic devices and electric vehicles capable of storing more energy and thus operating longer before they need to be recharged.
"For a long time till even today, the practical applicability of LMR cathode materials has been under continuous debate," Prof. Liu said.
"The focus of concern is whether the inherent drawbacks of voltage decay could be overcome, inspiring blooming research activities aiming at tackling the voltage decay problem and fundamentally understanding its origins. Our most notable achievement is successfully addressing the long-standing problem of voltage decay in LMRs. We discovered that by incorporating additional TMs into the cathode material, we could stabilize the honeycomb structure, resulting in a negligible voltage decay of only 0.02 mV per cycle."
The recent work by this team of researchers sheds light on the mechanisms underlying voltage decay in LMR cathodes, introducing a viable approach to minimize this issue. In the future, their cathode could be improved further or could inspire the design of other similar LMR cathode materials.
"While our current work has successfully tackled the voltage decay of LMRs, in the future we aim to address another crucial issue: the voltage hysteresis of LMRs," Prof. Liu said.
"The voltage hysteresis refers to the difference in voltage profiles during battery charging and discharging cycles. Previously, it was widely believed that the voltage hysteresis was due to the instability of the honeycomb superstructure, yet even with the improved stability during cycling brought by our 'cap' structure, the voltage hysteresis phenomenon has not been alleviated."
In their next studies, the researchers plan to conduct a thorough investigation of the mechanisms that might underpin voltage hysteresis in LMR cathodes. They hope that this will lead to important discoveries, which could inform the design of even better cathodes.
"Our next goal is to address the challenging issue of voltage hysteresis and further enhance the energy density of our cathode materials," Prof. Liu added. "We will also be searching for solutions to scale up the manufacturing process of our designed material for large-scale battery production. With these breakthroughs, we are getting one step closer to a future powered by efficient and sustainable energy storage."