Scientists at the University of New South Wales Sydney are working on ways to improve the efficiency and cost of hydrogen fuel cells, to increase access to clean fuel.
Hydrogen has been positioned as a key player in the race to a decarbonized future, but despite its potential, the road to commercialization has been a slow one.
There are several factors that scientists—including Professor Chuan Zhao, Dr. Quentin Meyer and Mr. Shiyang Liu from the School of Chemistry at UNSW—are trying to address, to increase the commercial viability of hydrogen fuel cells.
There are issues with the cost and resources of some of the key elements that make up a hydrogen fuel cell, including platinum, the material commonly used as the catalyst needed to activate the process. Creating alternatives to platinum catalysts is essential.
"Platinum is always going to be expensive, because there isn't a lot out there," says Prof. Zhao. "So, we need to explore alternatives, while also providing a quick and easy way to measure how well these new materials are working in hydrogen fuel cells."
In a study, published in Energy & Environmental Science, Prof. Zhao's team has developed a novel process to test the durability and stability of platinum alternatives that will supply new insights into cost-friendly options for hydrogen fuel cells.
The case for hydrogen fuel cells
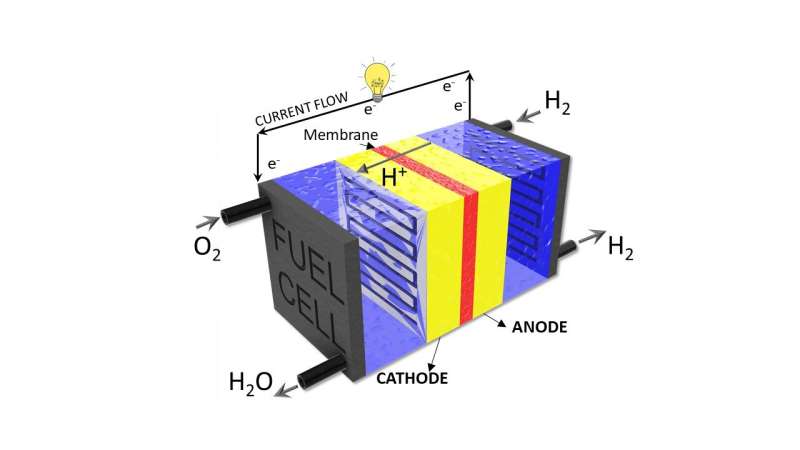
Hydrogen fuel cells, which were developed as a green energy source in the 19th century, use chemical reactions to break hydrogen into protons and electrons, producing electricity and water.
"Essentially you have two sides—an anode and a cathode. You put hydrogen on one side (the anode) and oxygen on the other (the cathode), and catalysts that make the two reactions happen," says Dr. Meyer. "One reaction is splitting hydrogen into protons and electrons, and then the other side oxidizing oxygen. The protons and electrons react with oxygen at the cathode to make water and electricity."
The key difference between hydrogen fuel cell technology and batteries is that you don't need to charge hydrogen fuel cells up. Instead of a petrol pump, you just have a hydrogen pump and it takes only three minutes to refuel a hydrogen fuel cell car.
The process is not only considered a clean source of energy, only producing water as a by-product, but it is also sustainable. Hydrogen itself is a very abundant element, and while it doesn't occur naturally, it can be extracted from water.
The problem of cost
"We are faced with a 'chicken or the egg' type problem, where we don't have enough hydrogen being processed, or enough places to use the hydrogen once it's been extracted," says Prof. Zhao. "So, as we start to produce more hydrogen and more fuel cells, then both will become cheaper."
Another key problem is the cost of the catalyst. The platinum which forms the essential middle layer of a fuel cell costs somewhere between $AUD 45,000 and $AUD 100,000 per kilo.
"One approach is to use platinum alternatives, such as iron, which only costs around $AUD0.1 per kilo " says Mr. Liu, "A particular promising material is Iron-nitrogen-carbon, also known as Fe-N-C."
However, these new platinum alternatives are not currently widely available because they are not as stable as platinum and break down at a faster rate in hydrogen fuel cells. "While platinum-based fuel cells can last up to 40,000 hours (about four and a half years), the iron-nitrogen-carbon materials can only run up to 300 hours (about two weeks), in a best-case-scenario," says Dr. Meyer.
Progress in the field has been slow, as finding alternatives and testing their durability is a lengthy and expensive process. "For instance, creating a new hydrogen fuel cell catalyst can take up to a year, and then even longer to understand exactly what's happening using expensive equipment that is hard to access," says Dr. Meyer.
Developing methods to analyze stability
For Prof. Zhao, Dr. Meyer and their team, the answer to addressing the existing issues in the field was to develop a method that allows you to understanding why some catalyst materials are not as stable as platinum.
"Using three novel methods that we tested in the lab, we can quickly figure out how stable our platinum-free fuel cell is and most importantly understand why. This approach can be easily adopted by scientists in other labs to gain quick and accurate insights into the efficiency of their fuel cells and catalysts," says Prof. Zhao.
Using these techniques, the team revealed that up to 75% of the iron-based active sites (the specific locations where the reactions happen) become inactive in the first 10 hours of running the fuel cell, due to the loss of iron active sites. This is then followed by carbon corrosion becoming the predominant degradation mechanism.
"This is particularly significant as we can pinpoint exactly what is happening and when it is happening. If we develop a material that has more stable active sites, we should see a slower decay in the first 10 hours, while carbon corrosion may have a similar trend," says Dr. Meyer.
"By allowing precise tracking of the degradation mechanisms, we expect that the research field will be able to make new materials targeting these stability issues. As a result, we believe our approach will help improve the stability of platinum-free catalysts and give this field a brighter future."
The next steps
While this is a major step in the field of hydrogen fuel cells, Prof. Zhao, Dr. Meyer, and their team have their sights set on the next goal.
"We are developing a catalyst where we're combining different metals to increase the stability of the catalysts," says Prof. Zhao. "Using the process we've developed here, we're able to get quick, reliable insights into the stability of these low-cost non-platinum catalysts. This gets us some exciting results understanding what is happening."
The team is also focusing on ways they can increase the scalability of the low-cost, platinum-free hydrogen fuel cell catalyst from the lab to a product that could be used to power real devices and, one day, power transport on our roads.