A truly solid, highly conductive electrolyte has been designed, bringing solid-state lithium batteries within reach. Batteries store energy chemically and rely on the movement of charged ions between a cathode and an anode, through an electrolyte. For most of battery history, this electrolyte has been a liquid, although research has long sought a solid alternative.
Polyethylene oxide (PEO)-based solid polymer electrolytes were developed in the 1970s, and have many attractive properties, including safety. Unlike today's lithium ion batteries, batteries with polymer electrolytes are less likely to burst into flames inside electric cars or in airplane holds if damaged.
Unfortunately, the ion conductivity at room temperature of polymer electrolytes is just too low to be practical. Other electrolytes that have recently been produced and described as "solid-state" actually contain gels.
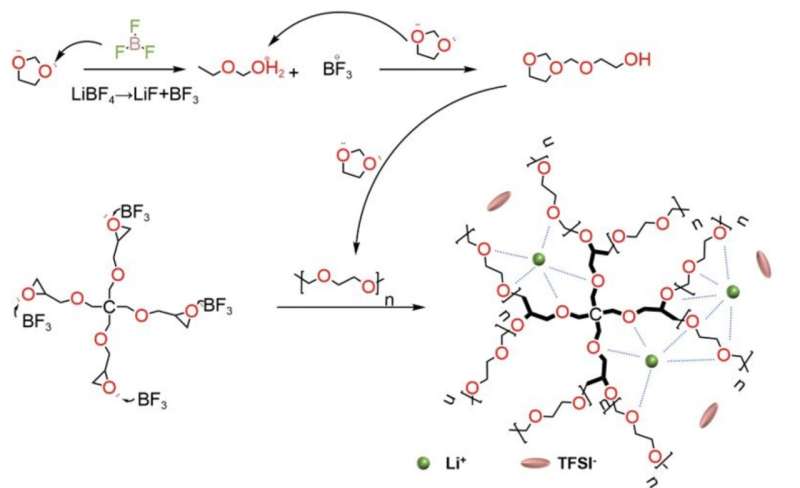
Quanfeng Dong and colleagues designed and synthesized a solid-state electrolyte from a cross-linked polymer composed of 1,3-dioxolane (DOL) and pentaerythritol glycidyl ether (PEG). The research is published in the journal PNAS Nexus.
This intrinsic polymer electrolyte (IPE) has a three-dimensional mesh structure, which has ionic conductivity up to 0.49 millisiemens per cm at room temperature—far higher than PEO. The intrinsic polymer electrolyte achieves lithium ion migration numbers of up to 0.85. Batteries built with intrinsic polymer electrolytes retain more than 90% of their storage capacity after 300 charge-discharge cycles.
The material may be a good choice for next-generation high energy-density all solid-state lithium-based batteries, according to the authors.