A research team led by Prof. Yan Lifeng from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) has designed a water-based nanomicellar electrolyte by using methylurea (Mu). The results were published in the Journal of the American Chemical Society.
Aqueous zinc ion batteries (AZIBs) are competitive candidates for clean energy storage, but they are severely limited by the irreversible electrochemical reaction of the zinc anode. Therefore, it is a crucial issue to explore how to regulate the electrochemical performance of AZIBs through electrolyte design optimization.
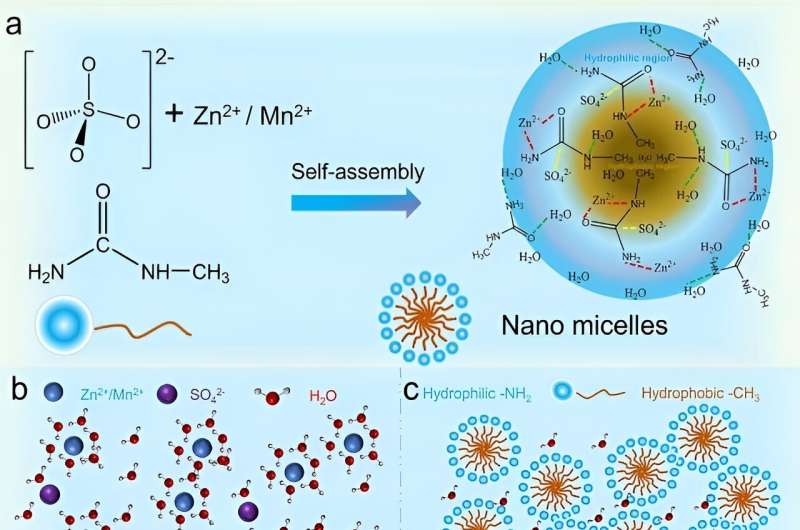
In this paper, the researchers proposed a unique design of nanomicellar electrolyte, which comprises ZnSO4, MnSO4 and a high concentration of Mu molecules through a self-assembly strategy, where the aqueous-solvent environment is partitioned into hydrophilic and hydrophobic regions, and cations and anions are encapsulated into nanodomains.
The nanoclusters blocked the consecutive water-based bulk hydrogen bonding network, breaking the hydrogen bonding network between water molecules and reconfiguring localized hydrogen bonding within the micelles and at the micelle /interface.
In addition, Mu molecules were involved in the solvent sheath structure of Zn2+/Mn2+ ions, thus inhibiting the water decomposition reaction. Zn2+/Mn2+ ions could be controllably released from the micellar clusters, diffused in a three-dimensional diffusion mode and uniformly deposited on the electrode surface.
Researchers also put forward a new solid-electrolyte interface (SEI) protective layer, Znx(Mu)ySO4∙nH2O, which was also converted in situ on the zinc anode surface to avoid zinc corrosion caused by the infiltration of water molecules.
The results of the various tests show that the carbonyl groups and on the Zn2+/Mn2+ and Mu molecules have a stronger binding capacity and are able to reduce the number of water molecules in the solvent sheath structure.
Thanks to the reconfiguration of hydrogen bonding inside the micellar electrolyte, highly reversible two-electron transition reactions were verified by non-in situ scanning electron microscopy, X-ray diffraction, Raman, X-ray diffraction and other test methods at different states of charge. Zinc-manganese batteries utilizing the two-electron reaction show an unprecedentedly high energy density of 800.4 Wh kg-1 (based on the cathode active material) and a discharge voltage of up to 1.87 V.
This work upgrades the previous understanding of the continuous solvent phase of the electrolyte and establishes a local/interfacial interaction network that effectively maintains the three-dimensional diffusive form of the ions and the favorable interfacial nucleation reaction, achieving effective suppression of metal dendrites and electrode side reactions.