The Li9N2Cl3 facilitates efficient lithium-ion transport due to its disordered lattice structure and presence of vacancies. Notably, it resists dendrite formation at 10 mA/cm2 and 10 mAh/cm2 due to its intrinsic lithium metal stability. Furthermore, it exhibits robust dry-air stability.
Incorporating this SSE in Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode-based all–solid-state batteries, we achieve substantial cycling stability (90.35% capacity retention over 1500 cycles at 0.5 C) and high areal capacity (4.8 mAh/cm2 in pouch cells). These findings pave the way for lithium metal batteries to meet electric vehicle performance demands.
—Li et al.
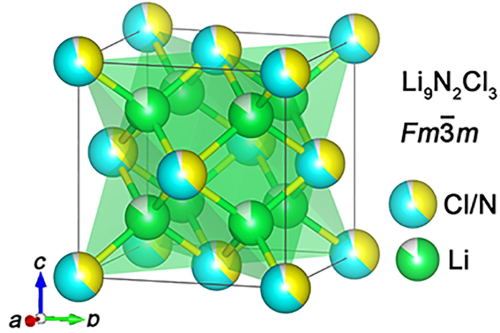
Calculated crystal structure of vacancy-rich Li9N2Cl3, Li+ (cyan), N3− (yellow), and Cl− (green). Li et al.