Underlying this favourable electrode combination is a rational electrolyte design based on 3.4 M LiFSI/FEMC featuring a shifted potential, which serves to aid formation of robust passivation layers on the anode and promote electrolyte stability against both reductive and oxidative degradations. Our electrolyte formulation offers a pathway towards both sustainable and high-performing LIBs, while the concept could be applied to other electrochemical energy technologies.
—Ko et al.
There are many reasons we want to transition away from using cobalt in order to improve lithium-ion batteries. For us the challenge is a technical one, but its impact could be environmental, economic, social and technological. We are pleased to report a new alternative to cobalt by using a novel combination of elements in the electrodes, including lithium, nickel, manganese, silicon and oxygen—all far more common and less problematic elements to produce and work with.
—Professor Atsuo Yamada, corresponding author
The new electrodes and electrolyte Yamada and his team created are not only devoid of cobalt, but they actually improve upon current battery chemistry in some ways. The new LIBs’ energy density is about 60% higher, which could equate to longer life, and it can deliver 4.4 volts, as opposed to about 3.2-3.7 volts of typical LIBs. But one of the most surprising technological achievements was to improve upon the recharge characteristics.
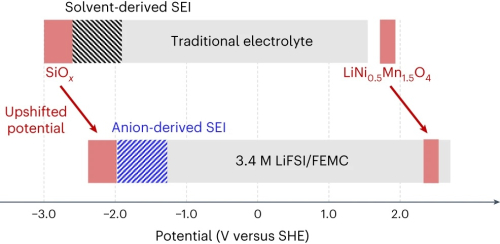
The optimized potential diagram of a highly sustainable high-energy-density battery system, combined with a high-capacity, Earth-abundant SiOx anode and a high-potential, Co-free spinel LiNi0.5Mn1.5O4 cathode in 3.4 M LiFSI/FEMC electrolyte. The stabilization mechanisms of the SiOx anode in 3.4 M LiFSI/FEMC include thermodynamic (upshifted potential) and kinetic (formation of SEI) factors. The upshifted electrode potential of SiOx aids in unburdening the kinetic support of the SEI, and, furthermore, the anion-derived SEI suppresses electrolyte decomposition more effectively. Notably, the upshifts in the electrode potentials of the SiOx anode and LiNi0.5Mn1.5O4 cathode are identical, thus maintaining the overall battery voltage while sustaining the electrode potential of the LiNi0.5Mn1.5O4 cathode within the potential window of 3.4 M LiFSI/FEMC. Ko et al.
Test batteries with the new chemistry were able to fully charge and discharge over 1,000 cycles (simulating three years of full use and charging), while only losing about 20% of their storage capacity.
We are delighted with the results so far, but getting here was not without its challenges. It was a struggle trying to suppress various undesirable reactions that were taking place in early versions of our new battery chemistries which could have drastically reduced the longevity of the batteries.
And we still have some way to go, as there are lingering minor reactions to mitigate in order to improve the safety and longevity even further. At present, we are confident that this research will lead to improved batteries for many applications, but some, where extreme durability and lifespan are required, might not be satisfied just yet.
—Professor Yamada
Although Yamada and his team were exploring applications in LIBs, the concepts that underlie their recent development can be applied to other electrochemical processes and devices, including other kinds of batteries, water splitting (to produce hydrogen and oxygen), ore smelting, electro-coating and more.