Bacteria that produce energy from inorganic carbon could have applications in sustainable biofuels.
Researchers have engineered Escherichia coli bacteria to make energy exclusively from carbon dioxide, according to a paper published today (November 27) in Cell.
E. coli are normally heterotrophs—organisms that get their energy sources from ingesting organic compounds, such as glucose—but the new study shows that they can be turned into autotrophs, making their own energy by turning carbon dioxide from the atmosphere into biomass.
“I find it fundamentally amazing that an organism which evolved over billions of years to live a heterotrophic lifestyle can so quickly and completely change into an autotroph,” Dave Savage, a biochemist at University of California, Berkeley, who was not involved with the study, tells The Scientist in an email. “It suggests that metabolism is extremely malleable.”
This process of using inorganic carbon to make biomass, called carbon fixation, could be used to solve “some of the biggest challenges of humanity today,” Ron Milo, a systems biologist at the Weizmann Institute of Science in Israel and the lead author of the paper, tells The Scientist. For example, increasing carbon fixation in plants generates more biomass, which could increase the world’s food supply.
The team set out to make E. coli—a “very genetically malleable model organism,” says Milo—fix carbon as a step toward sustainable industrial processes such as creating biofuel.
E. coli doesn’t normally have molecular mechanisms in place to use CO2, so the researchers gave it genes for the ability to fix carbon that were based on the gene sequence of carbon-fixing Pseudomonas bacteria. These changes weren’t enough to force the bacteria to switch to being autotrophic, so the team also disabled three genes involved in heterotrophic metabolism and put the bacteria into growth chambers with limited amounts of sugar, which starved them. In this environment, there was an advantage for bacteria that used CO2 instead of the finite sugar supply, and the researchers wanted to see if the bacteria could evolve to only use CO2.
The E. coli were grown on sodium formate, a carbon molecule that donates the necessary electrons during the process of making energy, but doesn’t contribute to biomass. The air in the growth chambers was also enriched with carbon dioxide.
After approximately 200 days, the bacteria relied completely on carbon dioxide from the air to generate biomass while taking in formate as a necessary ingredient for the chemical reactions. When the scientists analyzed the bacterial genome, they found that the bacteria evolved to use carbon dioxide as their energy source after as few as 11 mutations. Some of the changes occurred in genes related to carbon fixation, while others were in genes that are known to mutate in other lab evolution experiments or have no known role in energy production from CO2.
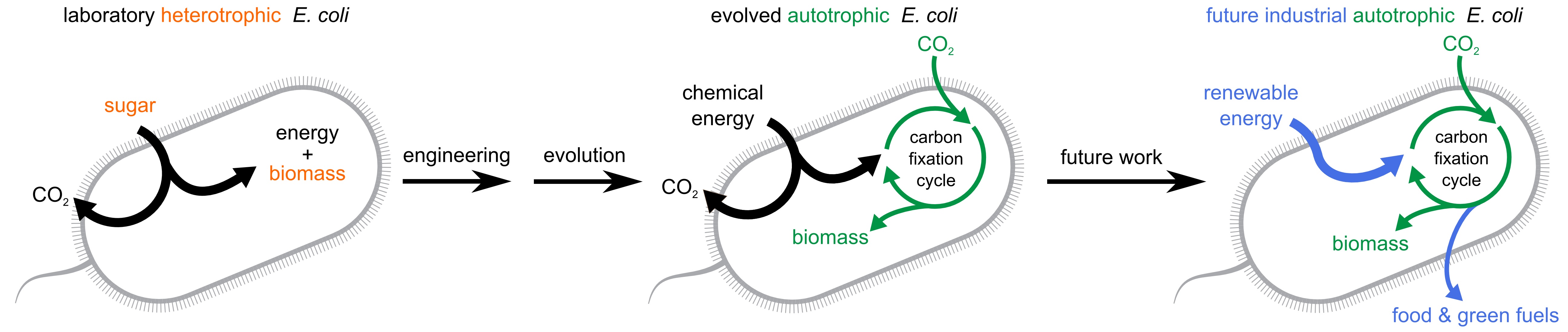 Heterotrophic E. coli (left) produce biomass from sugar, but lab-evolved autotrophic E. coli from the new study (center) use CO2 instead. The authors envision autotrophic E. coli that use renewable energy and have no net carbon emissions in the future (right).
Heterotrophic E. coli (left) produce biomass from sugar, but lab-evolved autotrophic E. coli from the new study (center) use CO2 instead. The authors envision autotrophic E. coli that use renewable energy and have no net carbon emissions in the future (right).
GLEIZER ET AL.“It’s a proof of concept for the field, that you can really rewire . . . the metabolic features of living organisms from scratch. It’s an exciting step forward,” Tobias Erb, a synthetic biologist at the Max Planck Institute for Terrestrial Microbiology in Germany who wrote a commentary on the study, tells The Scientist. However, “if the strain that they created [is] of biotechnological relevance in the future . . . I think is still up to debate,” he says.
For instance, the autotrophic E. coli currently produce more carbon dioxide as a byproduct than they take in. This could be solved by producing formate from carbon dioxide in the future, so that there are no net carbon dioxide emissions.
In addition, the researchers used high carbon dioxide levels in the bacteria’s growth chambers—around 10 percent of the air—but it’s only 0.04 percent of Earth’s atmosphere. “We’re interested to see if we could move it towards ambient carbon dioxide levels, meaning that one could use the ambient atmosphere that has much less [carbon dioxide], 400 parts per million,” says Milo.
“It’s an interesting concept now. Whether it actually is something that becomes useful in terms of application, that’s another question,” Patrik Jones, who studies microbial metabolic engineering at Imperial College London and was not involved with the study, tells The Scientist. “It’s definitely a step towards that direction . . . But then I think it’s important to realize that there are more steps needed in order to utilize this.”