Efficient and durable low-cost catalysts are essential for green hydrogen production and related chemical fuels production, both vital technologies for the transition to renewable energy. Research in this field increasingly focuses on metal exsolution reactions to fabricate catalysts with improved properties. A new study led by Forschungszentrum Jülich, in collaboration with international institutions, has unveiled how oxygen vacancies in oxide materials influence the stability of metal nanoparticles on the surface of such materials, which are critical to catalyst performance. The findings, published in Nature Communications, reveal practical strategies to enhance catalyst durability and make green hydrogen production more competitive.
Scientific Results
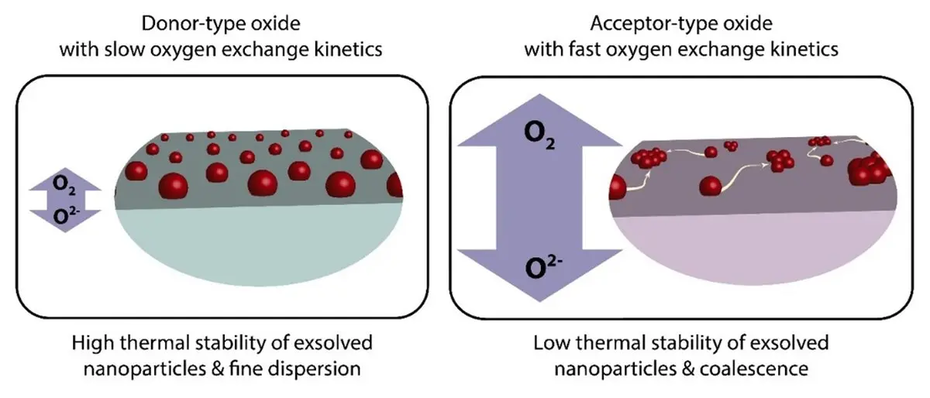
The study focused on the process of metal exsolution, a relatively new procedure where metal dopants initially part of the oxide lattice in oxide materials are released during thermal reduction to form nanoparticles on the oxide surface. These nanoparticles, in combination with the oxide substrate, create highly active interfaces that are crucial for catalyzing electrochemical reactions, such as water splitting for green hydrogen production.
The researchers demonstrate that oxygen vacancies—defects in the oxide crystal lattice where oxygen atoms are missing—play a pivotal role in nanoparticle stability. Oxides with high concentrations of oxygen vacancies that are used, for example, in fuel cells and electrolyzer cells, exhibit increased surface mobility of nanoparticles at elevated temperatures, which are typical for operation, causing them to coalesce into larger particles. This coalescence reduces the density of active sites, thereby diminishing the catalyst's efficiency. Conversely, oxides with lower concentrations of oxygen vacancies stabilize the nanoparticles, preventing coalescence and maintaining catalytic activity over time.
The team also identified a simple yet effective method to mitigate these effects. Introducing water vapor into the reaction environment slightly increases oxygen partial pressure, reducing the number of oxygen vacancies at the interface between the oxide and nanoparticles. This adjustment enhances nanoparticle stability and prolongs catalyst durability. Additionally, modifying the composition of the oxide material to inherently decrease oxygen vacancy concentration provides another viable approach for achieving long-term stability.
Social and Scientific Relevance
These findings have significant implications for the development of renewable energy systems. Exsolution catalysts are being discussed as promising candidates to replace conventional materials, particularly in solid oxide cells. Solid oxide cells are critical for both producing green hydrogen, an essential energy carrier for storage and transport, and converting it back into electricity at the highest efficiency levels. The durability of catalysts directly impacts the economic and operational feasibility of these devices.
Although metal exsolution reactions offer a promising approach for developing catalysts with enhanced properties, the limited durability of these catalysts—prone to structural and chemical degradation under operating conditions—remains a significant barrier to their practical application in green energy technologies. By addressing the issue of nanoparticle coalescence, this research could lead advance the viability of these novel catalysts.
Further Details
The research was a collaborative effort involving 20 scientists from institutions across Germany, the United States, and the United Kingdom. The study began during the collaborative doctoral project of lead author Dr. Moritz L. Weber at Forschungszentrum Jülich’s Peter Grünberg Institute (PGI-7) and Institute of Energy Materials and Devices (IMD-2), in collaboration with Imperial College London, and was supported by a DAAD scholarship. Dr. Weber continued his research as a Collaborative Postdoctoral Fellow at Lawrence Berkeley National Laboratory, working with experts at the Advanced Light Source and Dr. Felix Gunkel’s group at the PGI-7 in Jülich as well as Dr. Dylan Jennings from IMD-2 and colleagues at the Ernst Ruska-Centre for Microscopy and Spectroscopy with Electrons (ER-C) in Jülich.
The interdisciplinary nature of the study was essential for achieving its results, combining expertise in materials science, catalysis, and electrochemistry. Published in Nature Communications, the study provides actionable strategies for improving catalyst durability through adjustments in reaction conditions and material compositions and represents a significant step forward in the development of technologies for renewable energies.







