In a first, University of Sydney researchers have found evidence of how hydrogen causes embrittlement of steels. When hydrogen moves into steel, it makes the metal become brittle, leading to catastrophic failures. This has been one of the major challenges in moving towards a greener, hydrogen-fueled future, where steel tanks and pipelines are essential components that must be able to survive in pure hydrogen environments.
As described in a paper in Science, the researchers found that hydrogen accumulates at microstructures called dislocations and at the boundaries between the individual crystals that make up the steel. This accumulation weakens the steel along these features, leading to embrittlement.
Hydrogen embrittlement of high-strength steel is an obstacle for using these steels in sustainable energy production. Hydrogen embrittlement involves hydrogen-defect interactions at multiple-length scales. However, the challenge of measuring the precise location of hydrogen atoms limits our understanding. Thermal desorption spectroscopy can identify hydrogen retention or trapping, but data cannot be easily linked to the relative contributions of different microstructural features.
We used cryo-transfer atom probe tomography to observe hydrogen at specific microstructural features in steels. Direct observation of hydrogen at carbon-rich dislocations and grain boundaries provides validation for embrittlement models. Hydrogen observed at an incoherent interface between niobium carbides and the surrounding steel provides direct evidence that these incoherent boundaries can act as trapping sites. This information is vital for designing embrittlement-resistant steels.
—Chen et al.
 221147_web
221147_web 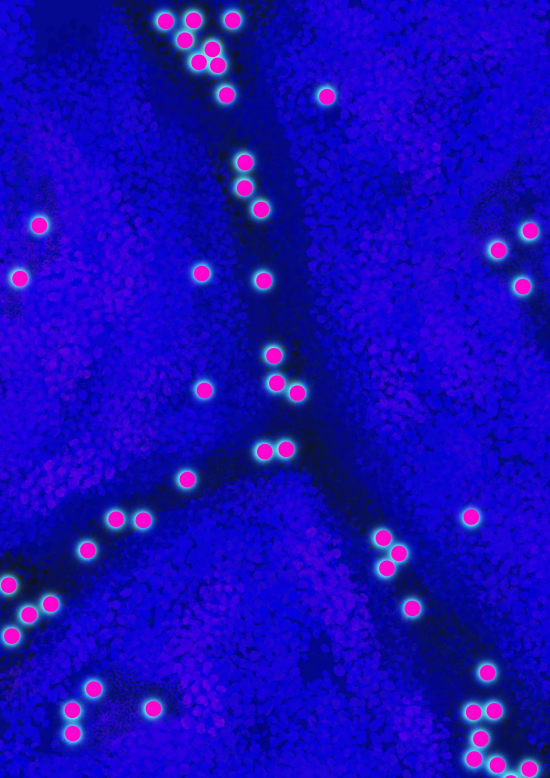 221147_web
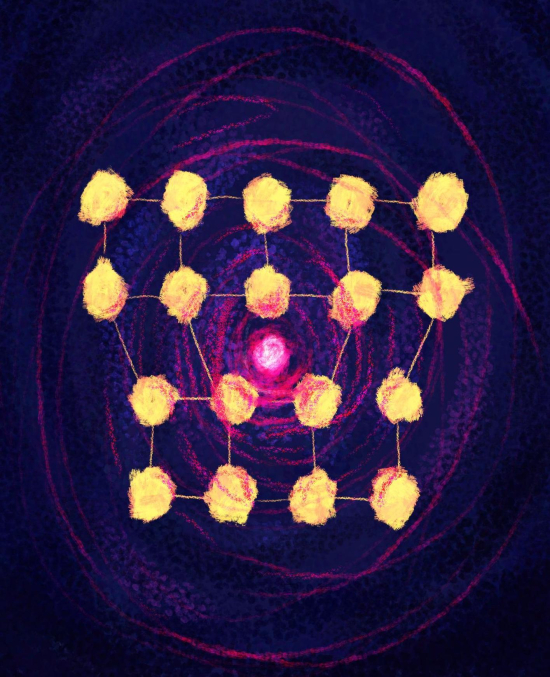
221147_web Top: Illustration highlighting the association of hydrogen (red) with dislocations in the crystal structure of steel. Bottom: Illustration highlighting the concentration of hydrogen atoms (red balls) at the crystal boundaries and dislocations in steel. Credit: University of Sydney
The researchers also found the first direct evidence that clusters of niobium carbide within the steel trap hydrogen in such a way that it cannot readily move to the dislocations and crystal boundaries to cause embrittlement. This effect has the potential to be used to design steels that can resist embrittlement.
Lead researcher Dr Yi-Sheng Chen from the Australian Centre for Microscopy and Microanalysis and Faculty of Engineering at the University of Sydney said these findings were an important step to finding a safe solution to produce, store and transport hydrogen.
These findings are vital for designing embrittlement-resistant steel; the carbides offer a solution to ensuring high-strength steels are not prone to early fracture and reduced toughness in the presence of hydrogen.
—Dr Chen
Senior author Professor Julie Cairney from the Australian Centre for Microscopy and Microanalysis and Faculty of Engineering at the University of Sydney said these findings were a positive step towards implementing clean fuels.
Hydrogen is a low carbon fuel source that could potentially replace fossil fuels. But there are challenges with the use of steel, the world's most important engineering material, to safely store and transport it. This research gives us key insights into how we might be able to improve this situation.
—Prof Cairney
Working in partnership with CITIC Metal, the researchers were able directly to observe hydrogen at microstructures in steels using Microscopy Australia’s state-of-the-art custom-designed cryogenic atom probe microscope.
 221149_web
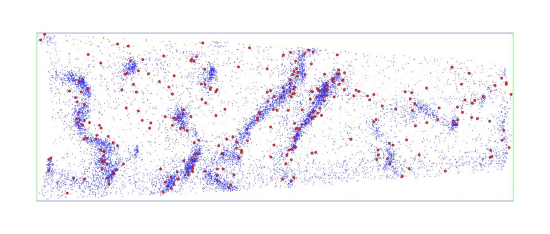
221149_web Atom probe image showing accumulations of hydrogen (red) at carbon-rich (blue) dislocations in steel. This evidence underpins the theoretical prediction of the origin of hydrogen embrittlement that limits the progress of hydrogen economy. Credit: University of Sydney