Direct methanol fuel cells (DMFCs) promise efficient energy generation with low emissions but the methane oxidation reaction (MOR) on which they rely is slow. While platinum is an effective catalyst widely used in fuel cells, it can be deactivated – or poisoned – by intermediate chemical species produced during the reaction. Alternatives based on gold are attractive, but like platinum, gold is too scarce and expensive for large-scale use in vehicles.
 Cross-sectional SEM images of mesoporous bimetallic Au-Ni films obtained from precursor solutions containing different Au:Ni molar ratios: (a) 100:0, (b) 75:25, (c) 50:50, and (d) 25:75 at an applied potential of -0.7?V and temperature of 40 degrees C.
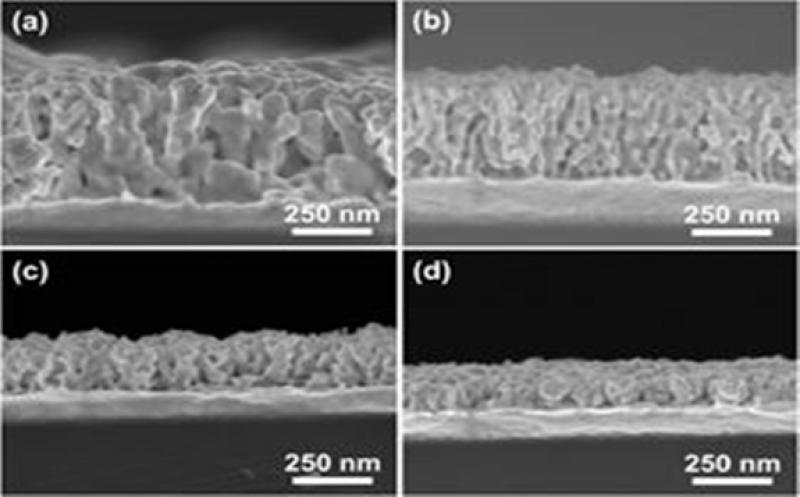
Cross-sectional SEM images of mesoporous bimetallic Au-Ni films obtained from precursor solutions containing different Au:Ni molar ratios: (a) 100:0, (b) 75:25, (c) 50:50, and (d) 25:75 at an applied potential of -0.7?V and temperature of 40 degrees C.
Now researchers have come up with a simple way of producing thin nanostructured films of gold and nickel, which reduce the overall amount of gold needed and offer better catalytic activity and stability.
“Gold has very interesting catalytic activity, whereby [it] is unreactive in bulk form but facilitates various oxidation reactions at low temperatures when nanostructured,” explains Yusuke Yamauchi of The University of Queensland, who led the work along with colleagues at Waseda University, National Institute for Materials Science in Japan, University of Wollongong, Tianjin University, Qingdao University of Science and Technology, and Kyung Hee University. “Gold it is expected to offer a new generation of catalysts that provides alternative choices to platinum, which does not have long term durability.”
Nanostructured – especially nanoporous – metals can be synthesized in various ways but typically require multi-step processes and harsh conditions. Instead, Yamauchi and his colleagues developed a ‘soft-templated’ approach during which metal precursors are electrochemically reduced in a solution containing polymer micelles. The micelles act as templates or molds for the formation of pores in the metallic films and can be easily removed afterwards with a solvent.
“Our approach is very simple and not time-consuming,” he says. “The simple preparation procedure [synthesizes] mesoporous bimetallic Au-Ni films with outstanding electrocatalytic activity for the MOR.”
The straightforward process yields well-mixed Au-Ni porous metallic films, with 28-nm-diameter pores that provide ample space for other chemical species to react.
“The metallic framework of this material is stable and highly conductive, which are beneficial for electrochemical applications,” points out Yamauchi. “Mesoporous bimetallic alloys are known to exhibit superior catalytic activity than their monometallic counterparts because of the synergistic effects arising from the modified chemical and electronic properties.”
The team believes that the high electrocatalytic activity exhibited by these Au-Ni porous metallic films for the MOR, which surpass other gold-based catalysts, make them highly suitable for DMFCs. The universal nature of the proposed method could be applied to a range of metals.
“The nanoarchitecture of this material also opens up a wide range of promising applications for the future in optics, energy storage, and biosensing,” adds Yamauchi. “This opportunity is significantly fascinating enough to be further explored.”







